Perspective | Open Access | Volume 8 (2): Article 44 | Published: 25 Jun 2025
COVID-19 pandemic experience in sub-Saharan Africa: the need to narrow the dichotomy between communicable and non-communicable diseases prevention
Samuel Onyinyechukwu Azubuike1,&
1Department of Public Health, Faculty of Health Sciences, National Open University of Nigeria
&Corresponding author: Samuel Onyinyechukwu Azubuike, Department of Public Health, Faculty of Health Sciences, National Open University of Nigeria, Email: samonaz2000@yahoo.com, sazubuike@noun.edu.ng
Received: 09 Dec 2024, Accepted: 25 Jun 2025, Published: 25 Jun 2025
Domain: Communicable and Non-Communicable Disease Epidemiology, Public Health
Keywords: COVID-19, Sub-Saharan Africa, Non-communicable diseases, Communicable diseases, Prevention
©Samuel Onyinyechukwu Azubuike et al Journal of Interventional Epidemiology and Public Health (ISSN: 2664-2824). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Samuel Onyinyechukwu Azubuike et al COVID-19 pandemic experience in sub-Saharan Africa: the need to narrow the dichotomy between communicable and non-communicable diseases prevention. Journal of Interventional Epidemiology and Public Health. 2025;8:44. https://doi.org/10.37432/jieph-d-24-02028
Abstract
Existing evidence suggests that pre-existing non-communicable diseases (NCDs) contributed significantly to the morbidity and mortality associated with COVID-19 infection in Africa. This underscores how the growing burden of communicable diseases in Africa could be complicated by the increasing burden of NCDs in the region. On the other hand, there are suggestions that the direct and indirect effects of communicable diseases such as COVID-19 infection could also complicate the existing burden of NCDs on the continent through their potential biological or socioeconomic disruptions. The available data on the COVID-19 pandemic experience in Africa suggests the need to re-evaluate the traditional dichotomy between communicable and non-communicable diseases’ preventive strategies in the region with the aim of adopting a more integrated approach where necessary. Policy frameworks aimed at preventing communicable diseases or managing large-scale outbreaks must account for their potential interplay with NCDs. Such an approach would facilitate coordinated efforts, foster partnerships, and enable the efficient mobilization of existing human and material resources toward the mutual prevention and management of both communicable and non-communicable diseases
Perspective
Introduction
Available data shows that more than 500 million cases of confirmed COVID-19 infections and above 6 million deaths were recorded globally by the beginning of June 2022. Of these figures, 1.7% of the cases and a disproportionate 2.4% of deaths occurred in Africa, while 41.9% cases and only 3.4% deaths occurred in Europe [1]. Although the pandemic has declined, it is necessary to understand the drivers of morbidity and mortality in the region for the purpose of the ongoing and future control programmes. The understanding of the drivers of morbidity and mortality associated with COVID-19 in the continent has evolved overtime. While the role of some factors implicated seems not to be very clear, that of others seem to be well established. For example, the role of extreme poverty and the overcrowded nature of African households on the occurrence and severity of the disease in the region remains a paradox, although emerging evidence suggests that they increase the risk of dying [2-4]
There is now sufficient data to suggest that pre-existing NCDs contributed to the severity of the infection in sub-Saharan Africa (SSA). For example, data from South Africa showed that 61% and 52% of the hospitalised COVID-19 patients had hypertension and diabetes respectively[5]. In the Democratic Republic of Congo, patients with NCDs comprised about 85% of all deaths[5] while in south west, Nigeria, cancer patients were 12 times more likely to die of COVID-19 infection (than non-cancer patients)[6]. In addition, the presence of diabetes, hypertension and renal diseases were noted as the commonest predictors of death in the Nigerian study[6]. Notably, the prevalence of pre-existing conditions such as cardiovascular diseases, cancer, and diabetes tends to increase with age. This suggests that these conditions may have complicated the increased risk of the COVID-19 among the older population. Incidentally, the elderly population of Africa is on the increase despite the population being predominantly young with the elderly (above 60 years) predicted to increase from 35 million in 2005 to 67 million by 2025 and 163 million by 2050 ( an estimated 218% increase between 2019-2050 has been projected [7-9]. The COVID-19 pandemic has, therefore, re-emphasised the potential dangers NCDs pose to the health and well-being of the continent presently and in the future. It could superimpose on the outbreak of communicable diseases as seen during the COVID-19 pandemic to wreak havoc on the population’s health and socioeconomic well-being. It is against this backdrop that this paper aims to highlight the impact of the burden of NCDs on the burden of COVID-19 infection in Africa (especially the sub-Saharan region) and vice versa, and the need to narrow the dichotomy between communicable and noncommunicable disease prevention, where necessary, in the region.
The paper was based on an analysis of COVID-19 and noncommunicable disease data extracted from the World Health Organisation website and other relevant publications available online. These data and information were critically reviewed, integrated, and appropriate conclusions were drawn, taking into consideration their epidemiological implications and potential relationships.
Non-communicable disease prevalence in Africa and its potential impact on the burden of COVID-19 in the region.
The burden of NCDs is on the rise in Africa, especially in SSA. A recent study showed an increase of 67% between 1990 (90·6 million DALYs (95% UI 81·0–101·9) and 2017 (151·3 million DALYs (133·4–171·8)[10]. It is worrisome that while the burden of NCDs showed a rapid increase, that of communicable, maternal, neonatal and nutritional diseases declined, with age-standardised NCDs (measured in DALY) closely trailing that of communicable diseases in SSA [10]. Similarly, WHO has observed that the age-standardised mortality rate of cancer in Africa is among the highest in the world ( > 650 per 100,000 compared to 438 per 100,000 in the WHO Region of the Americas)[11]. Besides, the risk of dying from the NCDs in Africa for both men and women aged between 30-70 years ( 20.6%) is higher than the global average (18%)[12]. The NCD burden in Africa is accounted for to a large extent by cardiovascular disease (17·9 million deaths annually)[13, 14] cancers (9·0 million), diabetes[(1·6 million)[14-1)] respiratory diseases(3·9 million )[13, 14] chronic kidney diseases[17], and mental health disorder[18], with the first three accounting for most cases.
Evidence from the COVID-19 pandemic showed that in spite of the low COVID-19 mortality rate in Africa compared to North America and Europe, the continent had the highest mortality rate among critically ill patients [19]. Although this could, in part, be attributed to other factors such as high prevalence of HIV/AIDS (especially in South Africa), the emergence of a more virulent strain, shortage and underutilisation of critical care resources (mechanical ventilator, oxygen supplies), lack of specialised staff to provide critical care treatment; the prevalence of NCDs among critically ill patents was undoubtedly a significant determinant of morbidity and mortality [19-22].
Moreover, the fact that the burden of some of these NCDs such as CVDs, diabetes and hypertension tend to be higher in younger age groups in SSA compared to the rest of the world (due to their early onset and possibly population structure)[23-25] suggests that communicable diseases like COVID-19 whose impact was aggravated by NCDs could take a higher toll on younger age group in SSA compared to the observations in high income countries. For instance, preliminary data suggested that while the higher proportion of COVID-19 cases among African men (63%) occurred among those aged 31-49 years, a greater proportion of cases among men in Europe was observed among those > 55 years of age[26, 27]. A similar observation was made in a Congolese study (mean age of 48 years compared to 54 years in the USA)[22]. The synergistic effect of NCDs and communicable diseases like COVID-19 could therefore have a negative implication for the socioeconomic well-being of families and countries in Africa, considering the age groups affected.
Notably, there is evidence that just as poverty and low socioeconomic status increase vulnerability to NCDs in low- and middle-income countries, NCDs also elevate the risk of becoming poor[28]. Incidentally, poverty remains the strongest driver of communicable disease prevalence across the globe, especially in SSA. Evidence of higher COVID-19-related mortality among low-income (compared with high income) communities in urban centres has been reported in South Africa and other parts of the world[29, 30]. It is, therefore, obvious that tackling NCDs and the socioeconomic variables associated with its prevalence in the region could invariably reduce the burden associated with outbreaks and prevalence of communicable diseases in the region. Hence, the prevalence of NCDs in the region should be taken into consideration when discussing the potential threat communicable disease outbreaks or pandemics such as COVID-19 pose to the health of the region. The pandemic should, therefore, provide an opportunity for increased awareness and the need for action (with respect to NCDS) given the direct and indirect threat it poses to the health and well-being of the continent.
Communicable diseases as a potential predisposing factor to NCDs and their severity in sub-Saharan Africa: Insights from the COVID-19 Pandemic
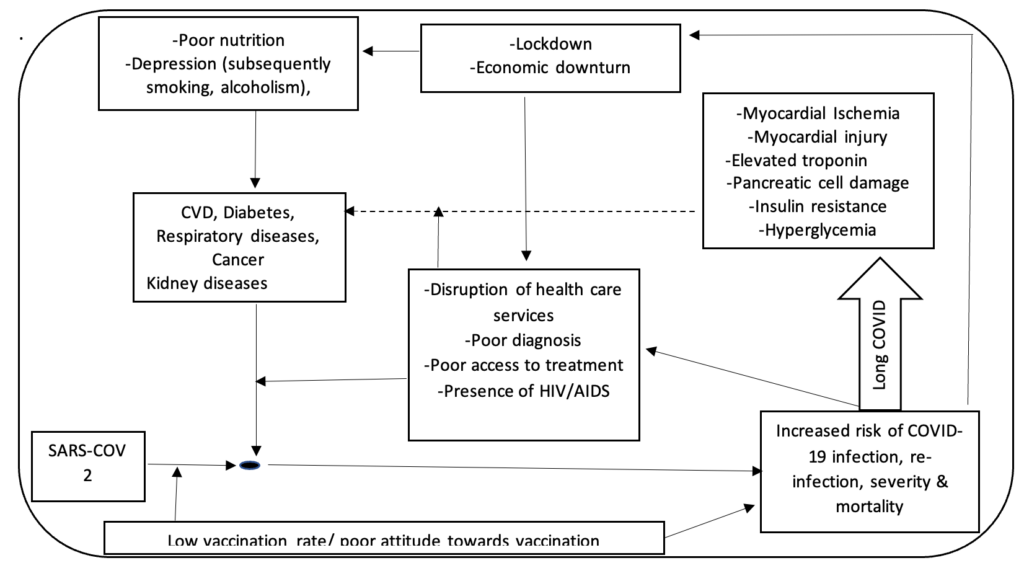
The COVID-19 pandemic has highlighted potential pathways through which communicable diseases could exacerbate existing NCD burden in the region or trigger new ones. For example, it has been suggested that COVID-19 has the potential to disrupt normal biological mechanisms in the body with potential adverse effects on NCDs [ 31]. The potential pathological relationship between COVID-19 infection and NCDs has been hypothesised to be mediated through the angiotensin converting enzyme 2 (ACE2) pathway, which provides an access door for SARS-COV-2 to enter human cells[32,33]. This enzyme is expressed in the heart, lungs, pancreatic cells, kidneys, liver and other organs[33, 34]. Through this pathway, the virus has been shown to be associated with multiple direct and indirect cardiovascular complications such as myocardial ischaemia, acute myocardial injury, myocarditis, cardiac arrhythmias, and venous thromboembolism[35]. For example, elevated troponin levels (associated with heart attack) or cardiac arrest were observed in 12% of COVID-19 patients without a history of CVD in China[35]. Moreover, insulin resistance, impaired insulin secretion, acute hyperglycaemia, pancreatic cell damage (which produces insulin needed for sugar metabolism), and beta cell autoimmunity (autoimmune diabetes) have also been associated with the infection[36,37]. There were also suggestions that it could lead to long-term lung damage, including pulmonary fibrosis. Although these were acute events, the potential long-term effects and implications for NCD incidence among patients, especially those who experienced long COVID in the region, deserve further investigation.
Furthermore, other potential indirect effects of large-scale communicable disease outbreaks similar to the COVID-19 pandemic will potentially complicate or increase vulnerability to NCDs. For example, health system disruption resulting from limited resources, time and manpower impeded the ability of NCD patients to access adequate treatment for their ailments during the current pandemic.[38]. A survey of 41 SSA countries by WHO in 2020 showed that more than 55% of the countries reported disruptions in the management of key NCDs such as hypertension and diabetic complications. In some places only emergency inpatient care for chronic diseases was available[5]. . Moreover, the economic downturn associated with communicable disease pandemics as seen in that of COVID-19 could have a substantial indirect negative effect on the burden of NCDs through its adverse impact on access to treatment, essential drugs, foods and capacity for individuals and families to cope with daily financial responsibilities. For example, a preliminary study by the World Bank on the socioeconomic impact of the COVID-19 pandemic among individuals and households in four SSA countries showed that 77% of the population live in households that have lost income due to the pandemic, while 20-25% of household in each country were unable to purchase essential medicines and staple foods[39]. A more recent report observed a 34% increase (since 2020) in the number of households who were unable to meet their basic food needs in West Africa alone[40]. This has peculiar implications for NCD patients, given that most of them require daily access to drugs to control their conditions and maintain a healthy living. Similarly, most NCD patients require specific dietary recommendations and foods which would be difficult to access in the event of financial hardship. Besides, the fact that the cost of health care in many African countries are borne by patients themselves has negative implications on the burden of diagnosed and undiagnosed NCDs[41]. This is because many of such patients or potential patients may find it difficult to secure medical appointments due to financial hardship. The inability of individuals to meet daily financial obligations, in addition to other adverse impacts of lockdowns (such as physical inactivity and frustrations), where applicable, has implications for mental health problems such as depression. Depression could increase vulnerability to alcoholism, obesity and smoking, which could further increase the risk of NCDs in the region. This, therefore, calls for a broader perspective, a more comprehensive approach and partnership towards disease prevention in the region. A hypothesised illustration of this relation based on the experience of the COVID-19 pandemic (Figure 1).
Conclusion
The COVID-19 pandemic experience in SSA calls for an appreciation of the fact that the direct and indirect effects of the increasing burden of NCDs in the region will complicate the existing burden of communicable diseases. Health policies towards the prevention and management of communicable diseases and large outbreaks should take that into consideration. On the other hand, the potential for large-scale communicable disease outbreaks such as COVID-19 to complicate the burden of key NCDs suggests the need to narrow the dichotomy between the prevention of both conditions. There is a need to encourage partnership (for example, in vaccination drives, research, improved access to health care, poverty reduction programmes, and risk factor awareness programmes) among teams implementing communicable and non-communicable disease programmes that hold mutual or complementary benefits. This will enhance both the effectiveness and efficiency of such programmes, given the limited resources to tackle the increasing burden of NCD amidst a significant burden of communicable diseases. The understanding of the potential relationship between communicable and non-communicable diseases can be harnessed towards the strengthening of community awareness and action against factors that predispose or increase vulnerability to communicable and non-communicable diseases. Finally, there is a need to review existing communicable and non-communicable disease programmes/policies in the region with the aim of integrating them where necessary. For example, consideration should be made towards integrating NCD prevention into primary health care programming.
References
- World Health Organization. WHO coronavirus (COVID-19) dashboard 2021 [Internet]. Geneva: World Health Organization; 2021 [cited 2025 Jun 25]. Available from: https://covid19.who.int/
- York G. Africa’s low COVID-19 death rate has multiple causes, WHO says [Internet]. The Globe and Mail. 2020 Sep 20 [cited 2025 Jun 25]. Available from: https://www.theglobeandmail.com/world/article-africas-low-covid-19-death-rate-has-multiple-causes-who-says/
- Spinney L. What can we learn from Africa’s experience of Covid? [Internet]. The Guardian. 2021 Feb 28 [cited 2025 Jun 25]. Available from: https://www.theguardian.com/world/2021/feb/28/what-can-we-learn-from-africa-experience-of-covid-death-toll-paradox
- Platt L, Warwick R. Are some ethnic groups more vulnerable to COVID-19 than others? [Internet]. London: Institute for Fiscal Studies; 2020 May [cited 2025 Jun]. Available from: https://www.ifs.org.uk/inequality/wp-content/uploads/2020/04/Are-some-ethnic-groups-more-vulnerable-to-COVID-19-than-others-V2-IFS-Briefing-Note.pdf
- World Health Organization Regional Office for Africa. Noncommunicable diseases increase risk of dying from COVID-19 in Africa [Internet]. Brazzaville: WHO Regional Office for Africa; 2020 Sep 10 [cited 2025 Jun 25]. Available from: https://www.afro.who.int/news/noncommunicable-diseases-increase-risk-dying-covid-19-africa
- Osibogun A, Balogun M, Abayomi A, Idris J, Kuyinu Y, Odukoya O, et al. Outcomes of COVID-19 patients with comorbidities in southwest Nigeria. Olusanya BO, editor. PLoS One. 2021 Mar 15;16(3):e0248281. Available from: https://dx.plos.org/10.1371/journal.pone.0248281 doi: 10.1371/journal.pone.0248281
- National Research Council (US) Panel on Policy Research and Data Needs to Meet the Challenge of Aging in Africa. Aging in Sub-Saharan Africa: recommendations for furthering research [Internet]. Washington (DC): National Academies Press (US); 2006 [cited 2025 Jun 25]. Available from: https://nap.nationalacademies.org/catalog/11708/aging-in-sub-saharan-africa-recommendations-for-furthering-research
- World Health Organization Regional Office for Africa. Ageing [Internet]. Brazzaville: WHO Regional Office for Africa; [cited 2025 Jun 25]. Available from: https://www.afro.who.int/health-topics/ageing
- Akinrolie O, Iwuagwu AO, Kalu ME, Rayner D, Oyinlola O, Ezulike CD, et al. Longitudinal studies of aging in Sub-Saharan Africa: review, limitations, and recommendations in preparation of projected aging population. Chukwuorji J, editor. Innov Aging. 2024 Jan 23;8(4):igae002. Available from: https://academic.oup.com/innovateage/article/doi/10.1093/geroni/igae002/7585958 doi: 10.1093/geroni/igae002
- Gouda HN, Charlson F, Sorsdahl K, Ahmadzada S, Ferrari AJ, Erskine H, et al. Burden of non-communicable diseases in Sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019 Oct;7(10):e1375-87. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214109X19303742 doi: 10.1016/S2214-109X(19)30374-2
- World Health Organization. Global status report on noncommunicable diseases 2014 [Internet]. Geneva: World Health Organization; 2014 [cited 2025 Jun 25]. Available from: https://iris.who.int/bitstream/handle/10665/148114/9789241564854_eng.pdf?sequence=1
- World Health Organization. World health statistics data visualizations dashboard: noncommunicable diseases and mental health data tables [Internet]. Geneva: World Health Organization; 2018 [cited 2025 Jun 25]. Available from: https://apps.who.int/gho/data/node.sdg
- Mensah G, Roth G, Sampson U, Moran A, Feigin V, Forouzanfar M, et al. Mortality from cardiovascular diseases in Sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013. Cardiovasc J Afr. 2015 Apr 30;26(2 Suppl 1):S6-10. Available from: http://www.cvja.co.za/onlinejournal/vol26/vol26_issue2_supplement/#8/z doi: 10.5830/CVJA-2015-036
- Bigna JJ, Noubiap JJ. The rising burden of non-communicable diseases in Sub-Saharan Africa. Lancet Glob Health. 2019 Sep 16;7(10):e1295-6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214109X19303705 doi: 10.1016/S2214-109X(19)30370-5
- NCD Risk Factor Collaboration (NCD-RisC) – Africa Working Group, Kengne AP, Bentham J, Zhou B, Peer N, Matsha TE, et al. Trends in obesity and diabetes across Africa from 1980 to 2014: an analysis of pooled population-based studies. Int J Epidemiol. 2017 Jun 4;46(5):1421-32. Available from: https://academic.oup.com/ije/article/46/5/1421/3861188 doi: 10.1093/ije/dyx078
- Atun R, Davies JI, Gale EAM, Bärnighausen T, Beran D, Kengne AP, et al. Diabetes in Sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol. 2017 Jul 5;5(8):622-67. Available from: https://linkinghub.elsevier.com/retrieve/pii/S221385871730181X doi: 10.1016/S2213-8587(17)30181-X
- Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, Naicker S, et al. The epidemiology of chronic kidney disease in Sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014 Feb 10;2(3):e174-81. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214109X14700026 doi: 10.1016/S2214-109X(14)70002-6
- Sankoh O, Sevalie S, Weston M. Mental health in Africa. Lancet Glob Health. 2018 Sep;6(9):e954-5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214109X18303036 doi: 10.1016/S2214-109X(18)30303-6
- Kirenga BJ, Byakika-Kibwika P. Excess COVID-19 mortality among critically ill patients in Africa. Lancet. 2021 May 22;397(10288):1860-1. Erratum in: Lancet. 2021 Jun 26;397(10288):1860-1. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673621005766 doi: 10.1016/S0140-6736(21)00576-6
- Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases, South Africa, Boulle A, Davies MA, Hussey H, Ismail M, Morden E, Vundle Z, et al. Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020 Aug 29;73(7):e2005-15. Available from: https://academic.oup.com/cid/article/73/7/e2005/5899044 doi: 10.1093/cid/ciaa1198
- Anjorin AA, Abioye AI, Asowata OE, Soipe A, Kazeem MI, Adesanya IO, et al. Comorbidities and the COVID-19 pandemic dynamics in Africa. Trop Med Int Health. 2020 Oct 4;26(1):2-13. Available from: https://onlinelibrary.wiley.com/doi/10.1111/tmi.13504 doi: 10.1111/tmi.13504
- Nachega JB, Ishoso DK, Otokoye JO, Hermans MP, Machekano RN, Sam-Agudu NA, et al. Clinical characteristics and outcomes of patients hospitalized for COVID-19 in Africa: early insights from the Democratic Republic of the Congo. Am J Trop Med Hyg. 2020 Oct 2;103(6):2419-28. Available from: https://ajtmh.org/doi/10.4269/ajtmh.20-1240 doi: 10.4269/ajtmh.20-1240
- Moran A, Forouzanfar M, Sampson U, Chugh S, Feigin V, Mensah G. The epidemiology of cardiovascular diseases in Sub-Saharan Africa: the Global Burden of Diseases, Injuries and Risk Factors 2010 Study. Prog Cardiovasc Dis. 2013 Sep 28;56(3):234-9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0033062013001722 doi: 10.1016/j.pcad.2013.09.019
- Kibirige D, Lumu W, Jones AG, Smeeth L, Hattersley AT, Nyirenda MJ. Understanding the manifestation of diabetes in Sub Saharan Africa to inform therapeutic approaches and preventive strategies: a narrative review. Clin Diabetes Endocrinol. 2019 Feb 14;5(1):2. Available from: https://clindiabetesendo.biomedcentral.com/articles/10.1186/s40842-019-0077-8 doi: 10.1186/s40842-019-0077-8
- World Health Organization. Hypertension [Internet]. Geneva: World Health Organization; 2023 Mar 16 [cited 2025 Jun 25]. Available from: https://www.who.int/news-room/fact-sheets/detail/hypertension
- World Health Organization. Situation update for the WHO African Region: External Situation Report 12 [Internet]. Geneva: World Health Organization; 2020 May 19 [cited 2025 Jun 25]. Available from: https://iris.who.int/bitstream/handle/10665/332150/SITREP_COVID-19_WHOAFRO_20200520-eng.pdf?sequence=1&isAllowed=y
- Lone SA, Ahmad A. COVID-19 pandemic – an African perspective. Emerg Microbes Infect. 2020 Jun 15;9(1):1300-8. Available from: https://www.tandfonline.com/doi/full/10.1080/22221751.2020.1775132 doi: 10.1080/22221751.2020.1775132
- Allen L, Williams J, Townsend N, Mikkelsen B, Roberts N, Foster C, et al. Socioeconomic status and non-communicable disease behavioural risk factors in low-income and lower-middle-income countries: a systematic review. Lancet Glob Health. 2017 Mar;5(3):e277-89. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214109X1730058X doi: 10.1016/S2214-109X(17)30058-X
- Hussey H, Zinyakatira N, Morden E, Ismail M, Paleker M, Bam JL, et al. Higher COVID-19 mortality in low-income communities in the City of Cape Town – a descriptive ecological study. Gates Open Res. 2021 Jun 4;5:90. Available from: https://gatesopenresearch.org/articles/5-90/v1 doi: 10.12688/gatesopenres.13288.1
- Mena GE, Martinez PP, Mahmud AS, Marquet PA, Buckee CO, Santillana M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science. 2021 Apr 27;372(6545):eabg5298. Available from: https://www.science.org/doi/10.1126/science.abg5298 doi: 10.1126/science.abg5298
- Gordon Patti K, Kohli P. Covid’s impact on non-communicable diseases: what we do not know may hurt us. Curr Cardiol Rep. 2022 May 7;24(7):829-37. Available from: https://link.springer.com/10.1007/s11886-022-01704-6 doi: 10.1007/s11886-022-01704-6
- Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al-Nasser AD. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020 Mar 20;9(3):231. Available from: https://www.mdpi.com/2076-0817/9/3/231 doi: 10.3390/pathogens9030231
- South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020 Apr 13;318(5):H1084-90. Available from: https://journals.physiology.org/doi/10.1152/ajpheart.00217.2020 doi: 10.1152/ajpheart.00217.2020
- Bindom SM, Lazartigues E. The sweeter side of ACE2: physiological evidence for a role in diabetes. Mol Cell Endocrinol. 2008 Oct 1;302(2):193-202. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0303720708004140 doi: 10.1016/j.mce.2008.09.020
- Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020 May;75(18):2352-71. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735109720346374 doi: 10.1016/j.jacc.2020.03.031
- Maddaloni E, Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020 Mar 31;36(7):e33213321. Available from: https://onlinelibrary.wiley.com/doi/10.1002/dmrr.3321 doi: 10.1002/dmrr.3321
- Hussain A, Bhowmik B, Do Vale Moreira NC. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020 Apr 9;162:108142. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168822720303922 doi: 10.1016/j.diabres.2020.108142
- World Health Organization. The impact of the COVID-19 pandemic on noncommunicable disease resources and services: results of a rapid assessment [Internet]. Geneva: World Health Organization; 2020 Sep 3 [cited 2025 Jun 25]. Available from: https://iris.who.int/bitstream/handle/10665/334136/9789240010291-eng.pdf?sequence=1
- Josephson A, Kilic T, Michler JD. Socioeconomic impacts of COVID-19 in four African countries [Internet]. Washington (DC): World Bank Group; 2020 Nov [cited 2025 Jun 25]. Available from: https://documents1.worldbank.org/curated/en/955251604433596591/pdf/Socioeconomic-Impacts-of-COVID-19-in-Four-African-Countries.pdf
- United Nations Economic Commission for Africa. Extreme hunger rises in West Africa due to COVID-19 pandemic [Internet]. Addis Ababa: United Nations Economic Commission for Africa; 2022 Jan 21 [cited 2025 Jun 25]. Available from: https://www.uneca.org/?q=stories/extreme-poverty-rises-in-west-africa-due-to-covid-19-pandemic
- Njagi P, Arsenijevic J, Groot W. Understanding variations in catastrophic health expenditure, its underlying determinants and impoverishment in Sub-Saharan African countries: a scoping review. Syst Rev. 2018 Sep 11;7(1):136. Available from: https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-018-0799-1 doi: 10.1186/s13643-018-0799-1
Menu, Tables and Figures
On Pubmed
On Google Scholar
Navigate this article
Figures


Keywords
- COVID-19
- Sub-Saharan Africa
- Non-communicable diseases
- Communicable diseases
- Prevention
