Research | Open Access | Volume 8 (3): Article 75 | Published: 16 Sep 2025
Factors associated with urogenital schistosomiasis in children in high-prevalence health posts in Tambacounda Department, Senegal
Menu, Tables and Figures
Navigate this article
Tables
| Variables | Absolute frequency (n) | Relative Frequency (%) |
|---|---|---|
| Distribution by district | ||
| Tambacounda | 65 | 31.71 |
| Maka Colibantang | 140 | 68.29 |
| Sex of the child | ||
| Male | 99 | 48.29 |
| Female | 106 | 51.71 |
| School attendance of the child | ||
| Yes | 25 | 12.20 |
| No | 180 | 87.80 |
| Age distribution (years) | ||
| 1–4 | 87 | 42.44 |
| 5–10 | 118 | 57.56 |
| Knowledge of urogenital schistosomiasis | ||
| Yes | 75 | 36.59 |
| No | 130 | 63.41 |
| Informed about urogenital schistosomiasis | ||
| Yes | 51 | 24.9 |
| No | 154 | 75.1 |
| Knowledge of the main symptoms | ||
| Yes | 59 | 28.8 |
| No | 146 | 71.2 |
| Knowledge of the transmission modes | ||
| Yes | 10 | 4.9 |
| No | 195 | 95.1 |
| Knowledge of curative treatment | ||
| Yes | 43 | 21 |
| No | 162 | 79 |
| Knowledge of the means of prevention | ||
| Yes | 14 | 6.8 |
| No | 191 | 93.2 |
| Would be willing to talk about illness | ||
| Yes | 155 | 75.61 |
| No | 50 | 24.39 |
| Talking to someone about the disease | ||
| Yes | 27 | 13.2 |
| No | 178 | 86.8 |
| Willingness to allow freshwater contact | ||
| Yes | 76 | 37.10 |
| No | 129 | 62.90 |
Table 1: Distribution of mothers/guardians according to socio-demographic characteristics, knowledge and attitudes towards urogenital schistosomiasis, Tambacounda, Senegal (n=205)
| Variables | Absolute frequency (n) | Relative Frequency (%) |
|---|---|---|
| Allows children contact with freshwater | ||
| Yes | 79 | 38.54 |
| No | 126 | 61.46 |
| Intention to seek care | ||
| Yes | 175 | 85.40 |
| No | 30 | 14.60 |
| Sought healthcare | ||
| Yes | 29 | 14.1 |
| No | 176 | 85.9 |
| Acceptability of preventive measures | ||
| Yes | 170 | 82.93 |
| No | 35 | 17.07 |
| History of hematuria | ||
| Yes | 43 | 21.00 |
| No | 162 | 79.00 |
| Hematuria at the time of investigation | ||
| Yes | 30 | 14.63 |
| No | 175 | 85.37 |
| Treated for schistosomiasis [5–10] years (N=118) | ||
| Yes | 108 | 91.53 |
| No | 10 | 8.47 |
| Treatment of schistosomiasis for [1–10] years | ||
| Yes | 108 | 52.70 |
| No | 97 | 47.30 |
| Reported side effects (N=108) | ||
| Yes | 4 | 3.70 |
| No | 104 | 96.3 |
| Urine dipstick results | ||
| Positive | 73 | 35.60 |
| Negative | 132 | 64.40 |
| Microscopy: Schistosoma haematobium eggs detection | ||
| Presence of eggs | 54 | 26.34 |
| Absence of eggs | 151 | 73.66 |
| Prevalence by age group (N=54) | ||
| 1–4 years | 13 | 14.94 |
| 5–10 years | 41 | 34.75 |
Table 2: Distribution of mothers or guardians according to practices and of children according to clinical, paraclinical and therapeutic characteristics, Tambacounda, Senegal (N=205)
| Variable | Urogenital Schistosomiasis Yes (n, %) | Urogenital Schistosomiasis No (n, %) | P value |
|---|---|---|---|
| District | |||
| Tambacounda | 32 (49.23%) | 33 (50.77%) | <0.001* |
| Maka Colibantang | 22 (15.71%) | 118 (84.29%) | |
| Age group (years) | |||
| 1–4 | 13 (14.94%) | 74 (85.06%) | 0.001* |
| 5–10 | 41 (34.75%) | 77 (65.25%) | |
| Sex of the child | |||
| Male | 31 (31.31%) | 68 (68.69%) | 0.153+ |
| Female | 23 (21.70%) | 83 (78.30%) | |
| School attendance of the child | |||
| Yes | 7 (28.00%) | 18 (72.00%) | 0.812 |
| No | 47 (26.11%) | 133 (73.89%) | |
| Knowledge of urogenital schistosomiasis | |||
| Yes | 23 (30.67%) | 52 (69.33%) | 0.324 |
| No | 31 (23.85%) | 99 (76.15%) | |
| Informed about urogenital schistosomiasis | |||
| Yes | 14 (27.45%) | 37 (72.55%) | 0.856 |
| No | 40 (25.97%) | 114 (74.03%) | |
| Knowledge of the main symptoms | |||
| Yes | 21 (35.59%) | 38 (64.41%) | 0.079+ |
| No | 33 (22.60%) | 113 (77.40%) | |
| Knowledge of transmission modes | |||
| Yes | 3 (30.00%) | 7 (70.00%) | 0.725 |
| No | 51 (26.15%) | 144 (73.85%) | |
| Knowledge of curative treatment | |||
| Yes | 14 (32.56%) | 29 (67.44%) | 0.332 |
| No | 40 (24.69%) | 122 (75.31%) | |
| Knowledge of prevention measures | |||
| Yes | 3 (21.43%) | 11 (78.57%) | 1.000 |
| No | 51 (26.70%) | 140 (73.30%) | |
| History of hematuria | |||
| Yes | 23 (53.49%) | 20 (46.51%) | <0.001* |
| No | 31 (19.14%) | 131 (80.86%) | |
| Talking to someone about the disease | |||
| Yes | 11 (40.74%) | 16 (59.26%) | 0.098 |
| No | 43 (24.16%) | 135 (75.84%) | |
| Sought healthcare | |||
| Yes | 14 (48.28%) | 15 (51.72%) | 0.006* |
| No | 40 (22.73%) | 136 (77.27%) | |
| Willingness to allow freshwater contact | |||
| Yes | 36 (47.37%) | 40 (52.63%) | <0.001* |
| No | 18 (13.95%) | 111 (86.05%) | |
| Acceptability of preventive measures | |||
| No | 15 (42.86%) | 20 (57.14%) | 0.020* |
| Yes | 39 (22.94%) | 131 (77.06%) | |
| Hematuria at the time of investigation | |||
| Yes | 22 (73.33%) | 8 (26.67%) | <0.001* |
| No | 32 (18.29%) | 143 (81.71%) | |
| Treated for schistosomiasis (all ages) | |||
| No | 36 (33.33%) | 72 (66.67%) | 0.018* |
| Yes | 18 (18.56%) | 79 (81.44%) | |
| Reported side effects (among treated only) | |||
| Yes | 3 (75.00%) | 1 (25.00%) | 0.019* |
| No | 15 (16.13%) | 78 (83.87%) | |
| Urine dipstick results | |||
| Positive | 50 (68.49%) | 23 (31.51%) | <0.001* |
| Negative | 4 (3.03%) | 128 (96.97%) |
Table 3: Identification of factors associated with urogenital schistosomiasis (UGS) among children aged 1 to 10 years old in bivariate analysis, Tambacounda, Senegal (n=205)
| Factors | P value (bivariate) | Crude OR | 95% CI | P value (multivariate) | Adjusted OR | 95% CI |
|---|---|---|---|---|---|---|
| District (Tambacounda vs Maka) | <0.001* | 5.20 | 2.67–10.12 | <0.001* | 18.99 | 3.66–98.53 |
| Age 5–10y vs 1–4y | 0.001* | 3.03 | 1.5–6.11 | — | — | — |
| Sex Male vs Female | 0.153 | 1.65 | 0.88–3.08 | — | — | — |
| Non-schooling vs Schooling | 0.812 | 0.91 | 0.36–2.31 | — | — | — |
| Knowledge of UGS (Yes vs No) | 0.324 | 1.41 | 0.75–2.67 | — | — | — |
| Informed about UGS (Yes vs No) | 0.856 | 1.08 | 0.53–2.2 | — | — | — |
| Knowledge symptoms (Yes vs No) | 0.079 | 1.89 | 0.98–3.66 | 0.010* | 8.71 | 2.56–29.58 |
| Knowledge transmission (Yes vs No) | 0.725 | 1.21 | 0.3–4.86 | — | — | — |
| Knowledge treatment (Yes vs No) | 0.332 | 1.47 | 0.71–3.06 | — | — | — |
| Knowledge prevention (Lack vs Yes) | 1.000 | 1.34 | 0.36–4.98 | — | — | — |
| Willingness to allow freshwater contact (Yes vs No) | <0.001* | 5.55 | 2.84–10.86 | — | — | — |
| Refusal of preventive measures (No acceptance vs Yes) | 0.020* | 2.52 | 1.18–5.38 | — | — | — |
| History of hematuria (Yes vs No) | <0.001* | 4.86 | 2.38–9.94 | <0.001* | 10.66 | 2.97–45.54 |
| Talking about disease (Yes vs No) | 0.098 | 2.16 | 0.93–5.0 | — | — | — |
| Sought healthcare (Yes vs No) | 0.006* | 3.17 | 1.41–7.13 | — | — | — |
| Hematuria during investigation (Yes vs No) | <0.001* | 12.29 | 5.02–30.09 | 0.014* | 17.38 | 1.28–235 |
| Not treated for UGS (No vs Yes) | 0.018* | 2.19 | 1.15–4.2 | — | — | — |
| Side effects exist (Yes vs No) | 0.019* | 15.60 | 1.52–160.29 | — | — | — |
| Urine dipstick Positive (vs Negative) | <0.001* | 69.57 | 22.9–211.29 | <0.001* | 199.00 | 27.44–723 |
aOR: Adjusted Odds Ratio, CI: Confidence Interval, *: Significant at the 5% level (p<0.05)
Table 4: Identification of factors associated with UGS among children aged 1 to 10 years old in multivariate analysis, Tambacounda, Senegal (n=205)
Figures

Keywords
- Schistosomiasis
- Urogenital
- Mothers
- Guardians
- Children
- Tambacounda
- Senegal
El Hadji Cheikh Abdoulaye Diop1,&, Mamadou Makhtar Mbacké Leye2, Ndèye Mbacké Kane3, Adélaïde Ndew Dog1, Bayal Cisse4, Dossolo Sanogo1
1Tambacounda Health District, Ministry of Health and Social Action, Senegal, 2Institute of Health and Development, Cheikh Anta Diop University of Dakar, Senegal, 3National Program for the Control of Neglected Tropical Diseases, Dakar, Senegal, 4Tambacounda Health Directorate, Ministry of Health and Social Action, Senegal
&Corresponding author: El Hadji Cheikh Abdoulaye DIOP, Email: docdiop82@gmail.com ORCID: https://orcid.org/0000-0001-9262-3739
Received: 29 May 2025, Accepted: 11 Sep 2025, Published: 16 Sep 2025
Domain: Neglected Tropical Diseases
Keywords: Schistosomiasis, Urogenital, Mothers, Guardians, Children, Tambacounda, Senegal
©El Hadji Cheikh Abdoulaye Diop et al. Journal of Interventional Epidemiology and Public Health (ISSN: 2664-2824). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: El Hadji Cheikh Abdoulaye Diop et al., Factors associated with urogenital schistosomiasis in children in high-prevalence health posts in Tambacounda Department, Senegal. Journal of Interventional Epidemiology and Public Health. 2025;8(3):75. https://doi.org/10.37432/jieph-d-25-00132
Abstract
Introduction: The prevalence of urogenital schistosomiasis remains very high (≥50%) in three health posts in the Tambacounda Department, despite mass treatment with Praziquantel among children aged 5–14 years. This study aims to determine the prevalence of urogenital schistosomiasis in high-endemicity health posts, to identify associated environmental and behavioral factors, and to assess the acceptability of recommended preventive measures among caregivers of children aged 1 to 10 years.
Methods: We conducted a descriptive analytical study in August 2024. The target population consisted of mothers or guardians of children aged 1 to 10 years. Data were collected using Kobo Toolbox and analyzed with R 4.4.1. Binary logistic regression was used to identify factors associated with urogenital schistosomiasis.
Results: The majority (82.93%) of mothers/guardians accepted preventive measures, while 14.63% of children presented with hematuria. Among children aged 5–10 years, 91.53% received treatment. Urine dipstick positivity was 35.6%, and 26.34% were carriers of Schistosoma haematobium (sh) eggs. The prevalence of urogenital schistosomiasis was 34.75% in children aged 5–10 years compared to 14.94% in those aged 1–5 years. Statistically significant associated factors included: residence in Tambacounda district (aOR=18.99, 95%CI :3.66–98.53; p <0.001), mothers/guardians knowledge of disease symptoms (aOR = 8.71, 95%CI : 2.56–29.58; p = 0.01), history of hematuria (aOR = 10.66, 95% CI : 2.97–45.54; p < 0.001), presence of hematuria at the time of the survey (aOR = 17.38, 95% CI : 1.28–235; p = 0.014) and positive urine dipstick results (aOR = 199, 95% CI: 27.44–723; p < 0.001).
Conclusion: Urogenital schistosomiasis prevalence remains high in three health posts in Tambacounda despite mass treatment in 5–15-year-old children. We recommend strengthening health education for mothers/guardians, systematic screening via urine dipsticks and extending mass treatment to include children aged 1–4 years.
Introduction
Urogenital schistosomiasis (also known as urinary bilharzia) is a parasitic disease caused by the blood fluke schistosoma haematobium (sh) that infects the urinary tract and surrounding tissues [1]. Transmission occurs primarily through contact with contaminated freshwater, where the parasite’s fork-tailed cercariae (infective larval stage) penetrate the skin, causing cercarial dermatitis [2]. During the chronic phase, symptoms include hematuria (blood in urine), pelvic pain, and recurrent urinary tract infections, potentially progressing to severe complications such as chronic bladder lesions [3]. Primarily endemic to sub-Saharan Africa, urogenital schistosomiasis affects over 700 million people across 78 countries, with 51 nations bearing the highest burden – accounting for approximately 90% of global cases and mortality [4,5]. Diagnosis of urogenital schistosomiasis is confirmed through microscopic urine analysis, while both therapeutic and preventive management primarily relies on praziquantel administration [3].
World Health Organization (WHO) classifies schistosomiasis endemicity levels as follows: low endemicity (prevalence ≤10%), moderate endemicity (prevalence 10-50%) and high endemicity (prevalence ≥50%)[6]. In Senegal, in 2013, mapping revealed that 59 out of 72 districts were endemic for schistosomiasis, with high endemicity in the Tambacounda and Kédougou regions. Despite annual Praziquantel distribution campaigns achieving over 80% coverage in these districts, the disease remains highly endemic [7]. In 2021, a study revealed an average schistosomiasis incidence rate of 28% at the Tambacounda Regional Hospital in 2017 [8].
In 2023, the impact assessment of urogenital schistosomiasis treatment revealed very high prevalence rates (≥50%) in three out of the 43 health facilities in the Tambacounda department: 91% in Balla Bani (Bohé Balédji Health Post), 75% in Ndiayéne (Ndoga Babacar Health Post), and 59% in Saré Gallo (Saré Ely Health Post), identified as “hotspots [9]. This persistence highlights the complexity of disease control and underscores the need to understand the underlying factors contributing to its high prevalence despite mass Praziquantel treatment. The sustained high transmission of schistosomiasis is exacerbated by factors such as poor hygiene, open-air urination practices [4,10], and disruptions caused by the COVID-19 pandemic, which reduced access to appropriate preventive treatments [11]. This situation underscores the urgent need to tailor prevention and control strategies to local specificities in order to enhance intervention effectiveness and better understand barriers to community acceptance of recommended preventive measures.
Although praziquantel is widely available, urogenital schistosomiasis continues to be frequently overlooked in Senegal. This is largely due to the limited diagnostic skills among healthcare workers and the underuse of effective detection methods, particularly in regions along the Senegal River basin where the disease is widespread [12,13]. In addition, many community members remain unaware of the condition, with some interpreting visible blood in urine as a normal sign of puberty, rather than a potential health issue [14]. These challenges highlight the need to strengthen both public awareness initiatives and diagnostic capabilities within local health services.
The study by Sacolo et al. (2018) reveals that while schistosomiasis is somewhat known in sub-Saharan Africa, misconceptions and risky behaviors persist. Populations often underestimate the disease when severe symptoms are absent. Barriers include limited healthcare access, cultural beliefs, and low treatment uptake. The authors recommend context-adapted strategies and community-based approaches to enhance prevention [15,16].
It is therefore crucial to assess the prevalence of urogenital schistosomiasis in health facilities identified as “hotspots,” with particular focus on the under 5 and 5–10 years age groups. Furthermore, it is equally important to identify the determinants and factors associated with this high prevalence, as well as to evaluate the acceptability of preventive measures against urogenital schistosomiasis among mothers or guardians of these children. Additionally, the acceptability of preventive measures would likely vary across health facilities and be influenced by awareness campaigns, cultural perceptions, and healthcare access in these different areas.
The objectives of the research are to determine the prevalence of urogenital schistosomiasis in “hotspot” health posts, to identify factors associated with this high prevalence, such as hygiene conditions and practices in freshwater areas, and to assess the acceptability of the recommended measures in place, taking into account awareness, cultural perceptions, and access to healthcare in these specific areas.
Methods
Study framework
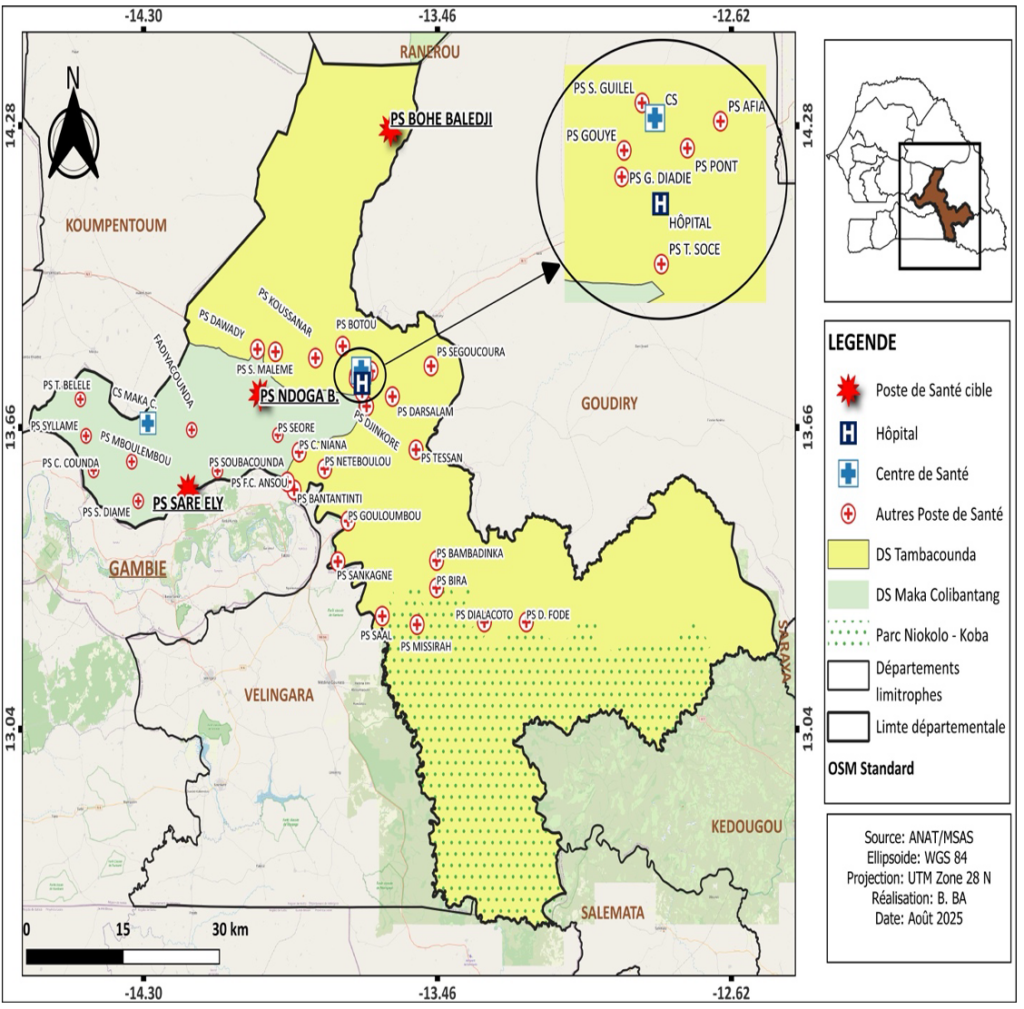
The department of Tambacounda, located in the eponymous region, had a population of 442,397 inhabitants in 2024, covering an area of 13,487 km², resulting in a population density of 33.32 inhabitants/km² (Figure 1). In 2024, the department had 2 health districts with 2 reference health posts (located in Tambacounda and Maka Colibantang) and 41 health posts [17]. This study focuses on three health posts in the department identified as “hotspots” due to their very high prevalence rates (≥ 50%) of urogenital schistosomiasis among children aged 5 to 14—the target group for Praziquantel treatment [9].
Study type, period, and population
We conducted a descriptive and analytical cross-sectional study in August 2024. The primary target population consisted of children aged 1 to 10 years living in the three “hotspot” health posts in the Tambacounda department: Ndoga Babacar and Saré Ely (Maka Colibantang district) and Bohé Balédji (Tambacounda district) [9]. For practical and ethical reasons, the study surveyed mothers or caregivers of children aged 1 to 10 years instead of the children themselves.
Inclusion and exclusion criteria
We included all children aged 1 to 10 years residing in the three target health posts’ catchment areas, whose mothers or caregivers provided free and informed consent.
We excluded children aged 1 to 10 years not residing in the target health posts’ service areas, those absent at the time of the study, cases of refusal (mother/guardian declined participation), and any child with circumstances preventing their participation (e.g., severe illness, disability affecting assessment).
Sampling
The sample size was calculated using Schwartz’s formula [18]
\[
\frac{Z^2 \cdot p \cdot (1 – p)}{e^2}
\]
where :
- Z = 1.96 (95% confidence level),
- p = 90% (estimated prevalence),
- and E = 5% (margin of error).
Accounting for a design effect of 2 and a 10% non-response rate, the final sample size retained for practical implementation is 307 participants.
The study employed a two-stage stratified cluster sampling design across three hotspot health posts (strata). For each post, four villages/neighborhoods were randomly selected (12 clusters in total). Each cluster targeted 24 children (12 boys and 12 girls), for a planned total of 288 participants, allocated equally across three non-overlapping age groups: 1–4 years, 5–10, and >10 years (i.e., 4 boys and 4 girls per age group within each cluster). Within each cluster, households were randomly selected, and all eligible participants present in the targeted age groups were included until the cluster allocation was reached. Data collection involved interviewing mothers/caregivers of children aged ≤10 years. This analysis focuses specifically on eight clusters (8 × 24 = 192 expected); in the field, 205 mothers/caregivers of children aged ≤10 years were included. This stratified approach ensured representation across key demographic groups while maintaining methodological rigor in these high-prevalence settings.
Data Collection
The responses were directly recorded on the forms, and each interview lasted approximately 20 to 30 minutes. On-site rapid urine tests (dipsticks) were performed by field investigators. Microscopic detection of haematobium eggs was carried out at the laboratory of the Tambacounda health center.
Operational definition of variables
The dependent variable was the presence of urogenital schistosomiasis, confirmed by the detection of schistosoma haematobium (sh) eggs using urine filtration microscopy [1,3]. Independent variables were grouped into:
Sociodemographic variables: include the child’s age (categorized as 1–5 and 5–10 years), sex (male/female), school attendance (yes/no), and district of residence (Tambacounda vs. Maka Colibantang).
Knowledge-related variables: assessed whether the respondent had heard of urogenital schistosomiasis, knew its main symptoms (e.g., hematuria, abdominal pain), modes of transmission (contact with contaminated water), curative treatment (praziquantel), and preventive measures (e.g., hygiene, avoiding freshwater contact).
Attitudinal variables: include willingness to talk about the disease and intention to seek healthcare if the child shows symptoms.
Practice-related variables: include whether the respondent allowed children to frequent freshwater environments (e.g., rivers, ponds), or whether the respondent encouraged behaviours that might expose children to contaminated water.
Clinical variables: include reported history of hematuria and presence of hematuria at the time of investigation (observed).
Paraclinical variables: include urine dipstick results (positive if hematuria is detected chemically) and microscopy results (presence or absence of S. haematobium eggs).
Therapeutic variables: include whether the child was treated for schistosomiasis in the past year, as well as any reported side effects of treatment.
Willingness to allow freshwater contact refers to the caregiver’s intention to let the child engage in activities such as bathing or playing in natural water sources.
Allows children contact with freshwater refers to the actual reported behaviour of letting children visit or use rivers, lakes, or ponds.
Data Analysis
The data were extracted from Kobo Collect and analysed using R software version 4.4.1. Descriptive analysis was used to assess knowledge, attitudes, practices (KAP), acceptability of preventive measures, as well as parasitological data and treatment follow-up. Factors associated with the presence of urogenital schistosomiasis were examined using Chi-square or Fisher’s exact tests in bivariate analysis. Binary logistic regression was performed using a stepwise method, where the addition of a new variable triggered a reassessment of previously included variables, potentially leading to their removal if they became non-significant. This approach identified factors associated with urogenital schistosomiasis at a significance threshold of 5% (α = 0.05).
Ethical considerations
The study protocol was approved by the National Committee for Health Research Ethics (CNERS) under reference number 179/MSAS/CNERS/SP on July 15, 2024 and received administrative authorization from the Directorate of Planning, Statistics, and Research under reference number 1062 on July 16, 2024. Investigators received training on ethical guidelines to ensure proper conduct throughout the study. Free and informed consent was obtained from mothers or caregivers after explaining the study’s objectives, procedures, potential risks, and benefits.
Results
Distribution of mothers/caregivers by sociodemographic characteristics, knowledge, and attitudes regarding urogenital schistosomiasis
We interviewed 205 mothers/caregivers. The majority of participants were from the Maka Colibantang district (68.29%), with a balanced distribution between boys (48.29%) and girls (51.71%). Most were unschooled (87.80%), and over half of the children were aged 5 to 10 (57.56%). Only 36.59% of participants were aware of urogenital schistosomiasis, and 24.9% had received information about the disease. Knowledge of symptoms (28.8%) and modes of transmission (4.9%) was low. Regarding curative treatment, 21% were aware of it, while knowledge of preventive measures remained marginal (6.8%). Nevertheless, 75.6% of participants were willing to discuss the disease, 85.4% expressed intent to seek healthcare, and 62.90% refused to let their children frequent freshwater sources (Table 1).
Distribution of mothers/caregivers by practices and children by clinical, paraclinical, and therapeutic characteristics
Among mothers/caregivers, 38.50% permitted their children to frequent freshwater sources, despite 21% reporting a history of hematuria. Only a minority (13.2%) had discussed the disease with anyone, and merely 14.1% sought healthcare. Acceptability of preventive measures was high (82.93%). At the time of the survey, 14.63% of children exhibited macroscopic hematuria. Among 5–10-year-olds, 91.53% received treatment for schistosomiasis, compared to 52.7% of children 10 years and under. Few participants (≈2% of the total sample; 4/205) reported side effects, corresponding to 3.7% among those treated (4/108). Urine dipstick tests were positive for 35.60% of participants, while microscopy confirmed Schistosoma haematobium (Sh) eggs in 26.34% of cases. Prevalence was higher in children aged 5–10 (34.75%) than in those under 5 (14.94%) (Table 2).
Factors associated with urogenital schistosomiasis
In bivariate analysis, urogenital schistosomiasis was significantly associated with residence in the Tambacounda district (cOR = 5.20, 95% CI: 2.67–10.12, p < 0.001), age 5 to 10 years (vs 1 to 4 years; cOR = 3.03, 95% CI: 1.50–6.11, p=0.001), freshwater contact (cOR = 5.55, 95% CI: 2.84–10.86, p<0.001), history of hematuria (cOR = 4.86, 95% CI: 2.38–9.94, p<0.001), current hematuria at the time of survey (cOR = 12.29, 95% CI: 5.02–30.09, p<0.001), rejection of preventive measures (cOR = 2.52, 95% CI: 1.18–5.38, p=0.020), healthcare-seeking (cOR = 3.17, 95% CI: 1.41–7.13, p=0.006), and a positive urine dipstick test (cOR = 69.57, 95% CI: 22.90–211.29, p<0.001) (Table III). Prior anti-schistosomal treatment was associated with infection (cOR = 2.19, 95% CI: 1.15–4.20, p=0.018). Discussing the disease with someone was not significantly associated (cOR=2.16, 95%CI : 0.93-5.0, p=0.098) (Table 3).
In binary logistic regression, the factors significantly associated with urogenital schistosomiasis were: residence in Tambacounda district (aOR = 18.99, 95% CI: 3.66-98.53; p < 0.001), children whose mothers/caregivers knew the disease symptoms (aOR = 8.71, 95% CI: 2.56-29.58; p = 0.01), history of hematuria (aOR = 10.66, 95% CI: 2.97-45.54; p < 0.001), presence of hematuria at time of survey (aOR = 17.38, 95% CI: 1.28-235; p = 0.014) and positive urine dipstick test (aOR = 199, 95% CI: 27.44-723; p < 0.001) (Table 4).
Discussion
The objective of this study was to determine the prevalence of Schistosoma haematobium infection among children aged 1-10 years. The prevalence was estimated at 26%, which, according to the World Health Organization (WHO) classification, corresponds to moderate endemicity. Multivariable analysis identified several factors independently associated with infection, including residence in the Tambacounda district, maternal knowledge of symptoms, a history of hematuria, the presence of hematuria at the time of investigation, and a positive urine dipstick test.
Knowledge about urogenital schistosomiasis was insufficient, a rate lower than that reported by Djagadou et al. in Togo in 2023 [19]. While most informed individuals recognised disease symptoms, understanding of transmission modes remained very limited, as was knowledge of prevention methods. Similar gaps were observed in Benin [20]. These findings underscore the necessity of strengthening awareness campaigns and health education to improve understanding of transmission and preventive strategies. Furthermore, Sidibé et al. (2024) emphasized that in highly endemic settings, the lack of knowledge is compounded by daily human-water contact and sociocultural perceptions, which significantly influence exposure to infected water and perpetuate disease transmission cycles [14].
Most participants were willing to discuss the disease and expressed willingness to seek healthcare for their children. A majority were reluctant to allow their children to frequent freshwater sources, demonstrating cautious awareness of the infection risks associated with these aquatic environments [21].
The study revealed that slightly more than a third of mothers/caregivers permitted their children to access freshwater sources, while 21% reported a history of hematuria in their children. In many rural communities, hematuria is often perceived as a natural phenomenon associated with male puberty rather than a clinical symptom of urogenital schistosomiasis [14]. This sociocultural interpretation contributes to the normalization of blood in urine and may delay or prevent appropriate health-seeking behavior. Such misconceptions represent a significant barrier to effective disease recognition and timely treatment, underscoring the need for culturally sensitive health education campaigns. These findings highlight how knowledge gaps, negative attitudes, and risky water-related practices contribute to schistosomiasis transmission, particularly among school-aged children and caregivers, emphasizing the urgent need for enhanced health education and addressing sociocultural barriers [21].
In our survey of children aged 1 to 10 years, the parasitological prevalence of Schistosoma haematobium eggs was 26.3%(urine dipstick positivity 35.6%), a level that classifies the department as moderately endemic according to WHO (10–50%) and indicates ongoing transmission despite mass drug administration (MDA) with praziquantel. This level is similar to the average incidence reported at the Tambacounda Regional Hospital in 2017 (28%) [8], but remains well below the ‘hotspots’ identified in 2023 at three health posts (≈ 59–91%) [9] underscoring the effectiveness of praziquantel mass drug administration (MDA) campaigns [22].
Our prevalence (26.3%) aligns with the moderate endemicity documented in Mauritania (25.8–35.6%) [23]. Conversely, Malian foci remain hyperendemic (69.2% among schoolchildren; up to 84.4% among PSAC), revealing fine‑scale heterogeneity and justifying micro‑targeted strategies combining optimized MDA, WASH and snail control, adaptive surveillance, and strengthened seasonal targeting [14].
Notably, infection rates were higher among 5 to 10 years olds compared to the under five year olds. This difference may be explained by increased mobility, autonomy, and participation in high-risk activities such as swimming or fetching water from natural sources, which are more frequent in older children. Agniwo et al. (2023) similarly reported that older children in Mali were more exposed due to frequent unsupervised contact with infested water, reinforcing the link between age-specific behaviors and elevated infection risk [24].
Our data confirm marked spatial heterogeneity of UGS. Living in the Tambacounda district markedly increases risk (aOR = 18.99; 95% CI: 3.66–98.5; p < 0.001). This magnitude aligns with programmatic analyses placing Tambacounda/Kédougou on annual treatment [25]. Comparable gradients exist elsewhere: in Togo (2022), Ogou versus Anié (aOR = 11.20; 95% CI: 5.2–24.0; p < 0.001) [26]. In The Gambia (2021), prevalence varied sharply (CRR 27.6%, 95% CI: 23.9–31.7; URR 12.0%, 95% CI: 9.4–15.1; LRR 0.6%, 95% CI: 0.2–1.8; NBR 0.0%, 95% CI: 0.0–0.8; p < 0.001). These geo-ecological foci reflect local hydrography, Bulinus spp., and water-use practices [27].
In our study, mothers’/guardians’ reported knowledge of symptoms was strongly associated with risk (aOR = 8.71, 95% CI: 2.56–29.58; p = 0.01). In endemic settings, this likely reflects reverse causality: parents “recognize” hematuria after having encountered it, rather than possessing preventive knowledge. Ghanaian evidence (2023, 2025) is mixed: in Pru-East, awareness of the disease (aOR = 0.02, 95% CI: 0.004–0.06; p < 0.001 [28] and of its transmission (aOR = 0.05, 95% CI: 0.01–0.22; p < 0.001) was protective, whereas elsewhere most respondents had never heard of schistosomiasis and risky practices persisted [28, 29].
A history of hematuria is a major predictor of Schistosoma haematobium infection (aOR = 10.66; 95% CI: 2.97–45.54; p < 0.001). In The Gambia, macroscopic and microscopic hematuria were strongly associated with egg excretion: macroscopic hematuria OR = 83.11 (95% CI: 10.47–659.49; p < 0.001) and microscopic hematuria OR = 22.43 (95% CI: 15.87–31.70; p < 0.001) [37]. In Nigeria, in a hyperendemic area, Balogun and al. reported that 99% of children with macroscopic hematuria excreted eggs; in Jidawa OR = 179.44 (95% CI: 10.70–3009.55; p = 0.001) and in Zobiya OR = 46.66 (95% CI: 6.18–352.40; p = 0.003) [25].
Having hematuria at the survey point greatly increased the odds of active Schistosoma haematobium infection (aOR = 17.38, 95% CI: 1.28–235; p = 0.014). This physiologically coherent signal has been reported across groups, including pregnant women in Richard-Toll (Senegal), where microscopic hematuria was significantly associated with infection [13]. The strength of this association generally increases with egg-excretion intensity, particularly for microscopic hematuria. That said, differential diagnoses (menses, urinary tract infections) can reduce specificity outside parasitic contexts, though among exposed populations the predictive value of hematuria remains high [30].
A positive urine reagent strip is very strongly associated with infection (aOR = 199; 95% CI: 27.44–723; p < 0.001). Grolimund CM et al. (2022) report high specificity (≈90–97%) and intensity-dependent sensitivity that approaches perfection beyond ~15 eggs/10 mL when “trace” is counted as positive [31]. Ethiopian studies corroborate this pattern, with ≈92% specificity but reduced sensitivity for light infections [32,33]. Co-including “clinical hematuria” and “reagent strip” may introduce collinearity and inflate the observed aOR [31,33].
Study limitations
Limitations of this study include potential recall and reporting bias among mothers/caregivers. The use of purposive sampling was limited to three high-prevalence health posts, and reliance on diagnostic tools (urine dipsticks and microscopy) may have underestimated the true infection prevalence. Furthermore, certain contextual factors, such as cultural practices, may not have been sufficiently accounted for, while the lack of longitudinal data limits our understanding of long-term transmission dynamics. Finally, the absence of control groups or intervention comparisons restricts the evaluation of preventive and therapeutic measures.
Conclusion
This study highlights significant gaps in knowledge and prevention of urogenital schistosomiasis among mothers/caregivers of children, as well as key environmental and behavioral factors associated with disease transmission. The findings underscore the urgent need to strengthen awareness campaigns and early screening programs to reduce schistosomiasis incidence in high-risk areas. Future interventions should incorporate targeted educational strategies, environmental control measures, continuous surveillance to address socio-cultural and environmental determinants of transmission, and expansion of mass Praziquantel treatment to children under 5 years. While this study provides crucial insights, its limitations must be acknowledged, and further research is needed to better understand transmission dynamics and optimise control strategies for urogenital schistosomiasis.
What is already known about the topic
- Urogenital schistosomiasis is endemic in sub-Saharan Africa, primarily due to exposure to contaminated water.
- Symptoms such as hematuria were key indicators, and insufficient knowledge about the disease contributed to its spread.
- Control strategies relied on antiparasitic drugs, with challenges related to reinfections and adherence to preventive measures.
What this study adds
- Study uncovered significant gaps in knowledge about urogenital schistosomiasis in Senegal and identifies specific risk factors, such as exposure to freshwater sources and children’s age.
- Demonstrated the effectiveness of urine test strips for screening and highlighted local disparities in risk.
Acknowledgements
We would like to thank the National Program for the Control of Neglected Tropical Diseases (PLMNT) and the Accelerating Resilient and Sustainable Elimination of Neglected Tropical Diseases (ARISE) project for funding this research. We also extend our sincere gratitude to all members of the extended core team of the Tambacounda Health District who participated in data collection, as well as to all the mothers and caregivers who kindly agreed to take part in this study.
Authors´ contributions
Dr. E.C.A. DIOP was the principal initiator of this study as part of his doctoral thesis in public health, supervised by Professor M.M.M. LEYE. A.N. DOG, Dr. D. SANOGO, and members of the Tambacounda District core team contributed to the questionnaire design. Dr. B. CISSE and Dr. N.M. KANE supported research funding.
| Variables | Absolute frequency (n) | Relative Frequency (%) |
|---|---|---|
| Distribution by district | ||
| Tambacounda | 65 | 31.71 |
| Maka Colibantang | 140 | 68.29 |
| Sex of the child | ||
| Male | 99 | 48.29 |
| Female | 106 | 51.71 |
| School attendance of the child | ||
| Yes | 25 | 12.20 |
| No | 180 | 87.80 |
| Age distribution (years) | ||
| 1–4 | 87 | 42.44 |
| 5–10 | 118 | 57.56 |
| Knowledge of urogenital schistosomiasis | ||
| Yes | 75 | 36.59 |
| No | 130 | 63.41 |
| Informed about urogenital schistosomiasis | ||
| Yes | 51 | 24.9 |
| No | 154 | 75.1 |
| Knowledge of the main symptoms | ||
| Yes | 59 | 28.8 |
| No | 146 | 71.2 |
| Knowledge of the transmission modes | ||
| Yes | 10 | 4.9 |
| No | 195 | 95.1 |
| Knowledge of curative treatment | ||
| Yes | 43 | 21 |
| No | 162 | 79 |
| Knowledge of the means of prevention | ||
| Yes | 14 | 6.8 |
| No | 191 | 93.2 |
| Would be willing to talk about illness | ||
| Yes | 155 | 75.61 |
| No | 50 | 24.39 |
| Talking to someone about the disease | ||
| Yes | 27 | 13.2 |
| No | 178 | 86.8 |
| Willingness to allow freshwater contact | ||
| Yes | 76 | 37.10 |
| No | 129 | 62.90 |
| Variables | Absolute frequency (n) | Relative Frequency (%) |
|---|---|---|
| Allows children contact with freshwater | ||
| Yes | 79 | 38.54 |
| No | 126 | 61.46 |
| Intention to seek care | ||
| Yes | 175 | 85.40 |
| No | 30 | 14.60 |
| Sought healthcare | ||
| Yes | 29 | 14.1 |
| No | 176 | 85.9 |
| Acceptability of preventive measures | ||
| Yes | 170 | 82.93 |
| No | 35 | 17.07 |
| History of hematuria | ||
| Yes | 43 | 21.00 |
| No | 162 | 79.00 |
| Hematuria at the time of investigation | ||
| Yes | 30 | 14.63 |
| No | 175 | 85.37 |
| Treated for schistosomiasis [5–10] years (N=118) | ||
| Yes | 108 | 91.53 |
| No | 10 | 8.47 |
| Treatment of schistosomiasis for [1–10] years | ||
| Yes | 108 | 52.70 |
| No | 97 | 47.30 |
| Reported side effects (N=108) | ||
| Yes | 4 | 3.70 |
| No | 104 | 96.3 |
| Urine dipstick results | ||
| Positive | 73 | 35.60 |
| Negative | 132 | 64.40 |
| Microscopy: Schistosoma haematobium eggs detection | ||
| Presence of eggs | 54 | 26.34 |
| Absence of eggs | 151 | 73.66 |
| Prevalence by age group (N=54) | ||
| 1–4 years | 13 | 14.94 |
| 5–10 years | 41 | 34.75 |
| Variable | Urogenital Schistosomiasis Yes (n, %) | Urogenital Schistosomiasis No (n, %) | P value |
|---|---|---|---|
| District | |||
| Tambacounda | 32 (49.23%) | 33 (50.77%) | <0.001* |
| Maka Colibantang | 22 (15.71%) | 118 (84.29%) | |
| Age group (years) | |||
| 1–4 | 13 (14.94%) | 74 (85.06%) | 0.001* |
| 5–10 | 41 (34.75%) | 77 (65.25%) | |
| Sex of the child | |||
| Male | 31 (31.31%) | 68 (68.69%) | 0.153+ |
| Female | 23 (21.70%) | 83 (78.30%) | |
| School attendance of the child | |||
| Yes | 7 (28.00%) | 18 (72.00%) | 0.812 |
| No | 47 (26.11%) | 133 (73.89%) | |
| Knowledge of urogenital schistosomiasis | |||
| Yes | 23 (30.67%) | 52 (69.33%) | 0.324 |
| No | 31 (23.85%) | 99 (76.15%) | |
| Informed about urogenital schistosomiasis | |||
| Yes | 14 (27.45%) | 37 (72.55%) | 0.856 |
| No | 40 (25.97%) | 114 (74.03%) | |
| Knowledge of the main symptoms | |||
| Yes | 21 (35.59%) | 38 (64.41%) | 0.079+ |
| No | 33 (22.60%) | 113 (77.40%) | |
| Knowledge of transmission modes | |||
| Yes | 3 (30.00%) | 7 (70.00%) | 0.725 |
| No | 51 (26.15%) | 144 (73.85%) | |
| Knowledge of curative treatment | |||
| Yes | 14 (32.56%) | 29 (67.44%) | 0.332 |
| No | 40 (24.69%) | 122 (75.31%) | |
| Knowledge of prevention measures | |||
| Yes | 3 (21.43%) | 11 (78.57%) | 1.000 |
| No | 51 (26.70%) | 140 (73.30%) | |
| History of hematuria | |||
| Yes | 23 (53.49%) | 20 (46.51%) | <0.001* |
| No | 31 (19.14%) | 131 (80.86%) | |
| Talking to someone about the disease | |||
| Yes | 11 (40.74%) | 16 (59.26%) | 0.098 |
| No | 43 (24.16%) | 135 (75.84%) | |
| Sought healthcare | |||
| Yes | 14 (48.28%) | 15 (51.72%) | 0.006* |
| No | 40 (22.73%) | 136 (77.27%) | |
| Willingness to allow freshwater contact | |||
| Yes | 36 (47.37%) | 40 (52.63%) | <0.001* |
| No | 18 (13.95%) | 111 (86.05%) | |
| Acceptability of preventive measures | |||
| No | 15 (42.86%) | 20 (57.14%) | 0.020* |
| Yes | 39 (22.94%) | 131 (77.06%) | |
| Hematuria at the time of investigation | |||
| Yes | 22 (73.33%) | 8 (26.67%) | <0.001* |
| No | 32 (18.29%) | 143 (81.71%) | |
| Treated for schistosomiasis (all ages) | |||
| No | 36 (33.33%) | 72 (66.67%) | 0.018* |
| Yes | 18 (18.56%) | 79 (81.44%) | |
| Reported side effects (among treated only) | |||
| Yes | 3 (75.00%) | 1 (25.00%) | 0.019* |
| No | 15 (16.13%) | 78 (83.87%) | |
| Urine dipstick results | |||
| Positive | 50 (68.49%) | 23 (31.51%) | <0.001* |
| Negative | 4 (3.03%) | 128 (96.97%) |
| Factors | P value (bivariate) | Crude OR | 95% CI | P value (multivariate) | Adjusted OR | 95% CI |
|---|---|---|---|---|---|---|
| District (Tambacounda vs Maka) | <0.001* | 5.20 | 2.67–10.12 | <0.001* | 18.99 | 3.66–98.53 |
| Age 5–10y vs 1–4y | 0.001* | 3.03 | 1.5–6.11 | — | — | — |
| Sex Male vs Female | 0.153 | 1.65 | 0.88–3.08 | — | — | — |
| Non-schooling vs Schooling | 0.812 | 0.91 | 0.36–2.31 | — | — | — |
| Knowledge of UGS (Yes vs No) | 0.324 | 1.41 | 0.75–2.67 | — | — | — |
| Informed about UGS (Yes vs No) | 0.856 | 1.08 | 0.53–2.2 | — | — | — |
| Knowledge symptoms (Yes vs No) | 0.079 | 1.89 | 0.98–3.66 | 0.010* | 8.71 | 2.56–29.58 |
| Knowledge transmission (Yes vs No) | 0.725 | 1.21 | 0.3–4.86 | — | — | — |
| Knowledge treatment (Yes vs No) | 0.332 | 1.47 | 0.71–3.06 | — | — | — |
| Knowledge prevention (Lack vs Yes) | 1.000 | 1.34 | 0.36–4.98 | — | — | — |
| Willingness to allow freshwater contact (Yes vs No) | <0.001* | 5.55 | 2.84–10.86 | — | — | — |
| Refusal of preventive measures (No acceptance vs Yes) | 0.020* | 2.52 | 1.18–5.38 | — | — | — |
| History of hematuria (Yes vs No) | <0.001* | 4.86 | 2.38–9.94 | <0.001* | 10.66 | 2.97–45.54 |
| Talking about disease (Yes vs No) | 0.098 | 2.16 | 0.93–5.0 | — | — | — |
| Sought healthcare (Yes vs No) | 0.006* | 3.17 | 1.41–7.13 | — | — | — |
| Hematuria during investigation (Yes vs No) | <0.001* | 12.29 | 5.02–30.09 | 0.014* | 17.38 | 1.28–235 |
| Not treated for UGS (No vs Yes) | 0.018* | 2.19 | 1.15–4.2 | — | — | — |
| Side effects exist (Yes vs No) | 0.019* | 15.60 | 1.52–160.29 | — | — | — |
| Urine dipstick Positive (vs Negative) | <0.001* | 69.57 | 22.9–211.29 | <0.001* | 199.00 | 27.44–723 |

References
- Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. The Lancet [Internet]. 2014 Jun 28 [cited 2025 Sep 12];383(9936):2253–64. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673613619492 https://doi.org/10.1016/S0140-6736(13)61949-2
- Ajana F, Baril L, Del Giudice P, Develoux M, et al. Bilharzioses ou schistosomoses. In: ePilly trop 2022 – Maladies infectieuses et tropicales. Paris: Collège des universitaires de Maladies Infectieuses et Tropicales (CMIT); 2022. p. 845-855. Available from: https://www.infectiologie.com/UserFiles/File/formation/epilly-trop/livre-epillytrop2022.pdf French Download livre-epillytrop2022.pdf
- Centers for Disease Control and Prevention (US). Schistosomiasis. Atlanta (GA): Centers for Disease Control and Prevention; 2024 Jul 7 [cited 2025 Sep 12]. [about 2 screens]. Available from: https://www.cdc.gov/dpdx/schistosomiasis/index.html
- World Health Organization. Schistosomiasis [Internet]. Geneva (Switzerland): World Health Organization; 2023 Jan 1 [cited 2025 Sep 12]. [about 4 screens]. Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- World Health Organization. Schistosomiasis (Bilharzia) [Internet]. Geneva (Switzerland): World Health Organization; 2025 [cited 2025 Sep 12]. [about 1 screen]. Available from: https://www.who.int/health-topics/schistosomiasis#tab=tab_1
- World Health Organization. WHO guideline on control and elimination of human schistosomiasis. Geneva (Switzerland): World Health Organization; 2022 Feb 14 [cited 2025 Sep 12]. 142 p. Available from: https://www.who.int/publications/i/item/9789240041608 Download 9789240041608-eng.pdf
- Ministère de la Santé et de l’Action Sociale (Sénégal). Plan national stratégique de lutte contre les maladies tropicales négligées [National strategic plan for the fight against neglected tropical diseases]. Dakar (Sénégal): Ministère de la Santé et de l’Action Sociale; 2020 [cited 2025 Sep 15]. 97 p. Available from: https://espen.afro.who.int/sites/default/files/content/document/Senegal%20Plan%20National%20stratégique%20MTN%202022-2025.pdf Download Sénégal Plan National stratégique MTN 2022-2025.pdf
- KA Oumar. Incidence de la bilharziose urogénitale au centre hospitalier de Tambacounda de 2015 à 2017 [Incidence of urogenital bilharzia at the Tambacounda hospital center from 2015 to 2017] [dissertation] [Internet]. [Dakar (Sénégal)]: Université Cheikh Anta Diop; 2020 [cited 2025 Sep 15]. 62 p. French
- Diop B, Sylla K, Kane NdMb, Boh OK, Guèye B, Ba M, Talla I, Mané M, Monteil R, Kinvi B, Zoure HGM, Ortega JC, Mwinzi P, Sacko M, Faye B. Schistosomiasis control in Senegal: results from community data analysis for optimizing preventive chemotherapy intervention with praziquantel. Infect Dis Poverty [Internet]. 2023 Nov 27 [cited 2025 Sep 17];12(1):106. Available from: https://idpjournal.biomedcentral.com/articles/10.1186/s40249-023-01155-3 https://doi.org/10.1186/s40249-023-01155-3
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. The Lancet Infectious Diseases [Internet]. 2006 Jul [cited 2025 Sep 12];6(7):411–25. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1473309906705217 https://doi.org/10.1016/S1473-3099(06)70521-7
- Toor J, Adams ER, Aliee M, Amoah B, Anderson RM, Ayabina D, Bailey R, Basáñez MG, Blok DJ, Blumberg S, Borlase A, Rivera RC, Castaño MS, Chitnis N, Coffeng LE, Crump RE, Das A, Davis CN, Davis EL, Deiner MS, Diggle PJ, Fronterre C, Giardina F, Giorgi E, Graham M, Hamley JID, Huang CI, Kura K, Lietman TM, Lucas TCD, Malizia V, Medley GF, Meeyai A, Michael E, Porco TC, Prada JM, Rock KS, Le Rutte EA, Smith ME, Spencer SEF, Stolk WA, Touloupou P, Vasconcelos A, Vegvari C, De Vlas SJ, Walker M, Hollingsworth TD. Predicted impact of covid-19 on neglected tropical disease programs and the opportunity for innovation. Clinical Infectious Diseases [Internet]. 2021 Apr 26 [cited 2025 Sep 12];72(8):1463–6. Available from: https://academic.oup.com/cid/article/72/8/1463/5912106 https://doi.org/10.1093/cid/ciaa933
- Aula OP, McManus DP, Jones MK, Gordon CA. Schistosomiasis with a focus on Africa. TropicalMed [Internet]. 2021 Jun 22 [cited 2025 Sep 12];6(3):109. Available from: https://www.mdpi.com/2414-6366/6/3/109 https://doi.org/10.3390/tropicalmed6030109
- Ndiour CN, Senghor B, Thiam O, Niang S, Wotodjo AN, Faye BT, Ndiaye NA, Sow O, Sylla K, Ndiaye M, Gaye O, Faye B, Sokhna C, Doucouré S, Sow D. Prevalence and associated factors of schistosomiasis among pregnant women in northern Senegal. BMC Infect Dis [Internet]. 2024 Jul 9 [cited 2025 Sep 12];24(1):682. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-024-09443-5 https://doi.org/10.1186/s12879-024-09443-5
- Sidibé B, Agniwo P, Diakité A, Savassi BAE, Bolodji S, Doumbo SN, Akplogan A, Guindo H, Ibikounlé M, Dembélé L, Djimde A, Boissier J, Dabo A. Human-water interactions associated to cercarial emergence pattern and their influences on urinary schistosomiasis transmission in two endemic areas in Mali. Infect Dis Poverty [Internet]. 2024 Aug 29 [cited 2025 Sep 12];13(1):62. Available from: https://idpjournal.biomedcentral.com/articles/10.1186/s40249-024-01229-w https://doi.org/10.1186/s40249-024-01229-w
- Sacolo H, Chimbari M, Kalinda C. Knowledge, attitudes and practices on Schistosomiasis in sub-Saharan Africa: a systematic review. BMC Infect Dis [Internet]. 2018 Jan 18 [cited 2025 Sep 13];18(1):46. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-017-2923-6 https://doi.org/10.1186/s12879-017-2923-6
- Sacolo-Gwebu H, Kabuyaya M, Chimbari M. Knowledge, attitudes and practices on schistosomiasis and soil-transmitted helminths among caregivers in Ingwavuma area in uMkhanyakude district, South Africa. BMC Infect Dis [Internet]. 2019 Dec [cited 2025 Sep 13];19(1):734. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-019-4253-3 https://doi.org/10.1186/s12879-019-4253-3
- Agence Nationale de la Statistique et de la Démographie (Sénégal). Situation économique et sociale régionale Tambacounda 2022-2023 [Regional economic and social situation in Tambacounda 2022-2023] [Internet]. Dakar (Senegal): Agence Nationale de la Statistique et de la Démographie; 2025 Apr [cited 2025 Sep 13]. 141 p. Available from: https://www.ansd.sn/sites/default/files/2025-05/SES-Tambacounda_2022-2023.pdf French Download SES-Tambacounda_2022-2023.pdf
- Schwartz D, Flamant R, Lellouch J. Méthodologie: évaluation des actions de santé. Paris: Flammarion Médecine-Sciences; 1995.
- Djagadou KA, Tchamdja T, Némi KD, Balaka A, Djibril MA. Connaissances, attitudes et pratiques des populations de la ville de Lomé en matière de prévention de la bilharziose: cas du canton de Légbassito. Pan Afr Med J [Internet]. 2019 Sep 10 [cited 2025 Sep 15];34. Available from: http://www.panafrican-med-journal.com/content/article/34/19/full/ https://doi.org/10.11604/pamj.2019.34.19.18918
- Agossoukpe BS, Tognon H, Daho JY, Adegbola P, Tokpanoude CNI, Djossou ESE, Abgrene K, Soubeiga D. Urinary schistosomiasis: factors associated with modern care research by the community of Lanta in Benin in 2023. OALib [Internet]. 2023 [cited 2025 Sep 15];10(12):1–13. Available from: http://www.oalib.com/paper/pdf/6812493 https://doi.org/10.4236/oalib.1111074
- Green AE, Anchang-Kimbi JK, Wepnje GB, Ndassi VD, Kimbi HK. Distribution and factors associated with urogenital schistosomiasis in the Tiko Health District, a semi-urban setting, South West Region, Cameroon. Infect Dis Poverty [Internet]. 2021 Apr 12 [cited 2025 Sep 16];10(1):49. Available from: https://idpjournal.biomedcentral.com/articles/10.1186/s40249-021-00827-2 https://doi.org/10.1186/s40249-021-00827-2
- Kokaliaris C, Garba A, Matuska M, Bronzan RN, Colley DG, Dorkenoo AM, Ekpo UF, Fleming FM, French MD, Kabore A, Mbonigaba JB, Midzi N, Mwinzi PNM, N’Goran EK, Polo MR, Sacko M, Tchuem Tchuenté LA, Tukahebwa EM, Uvon PA, Yang G, Wiesner L, Zhang Y, Utzinger J, Vounatsou P. Effect of preventive chemotherapy with praziquantel on schistosomiasis among school-aged children in sub-Saharan Africa: a spatiotemporal modelling study. The Lancet Infectious Diseases [Internet]. 2022 Jan [cited 2025 Sep 16];22(1):136–49. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1473309921000906 https://doi.org/10.1016/S1473-3099(21)00090-6
- Nakatt L, Gaye PM, Moukah MO, Niang B, Basco L, Ranque S, Ould Mohamed Salem Boukhary A. Urogenital schistosomiasis in schoolchildren in the lake zones of Kankossa and Oued Rawdha, southern Mauritania: The first parasitological and malacological survey. PLoS Negl Trop Dis [Internet]. 2024 Sep 25 [cited 2025 Sep 16];18(9):e0012505. Available from: https://dx.plos.org/10.1371/journal.pntd.0012505 https://doi.org/10.1371/journal.pntd.0012505
- Agniwo P, Sidibé B, Diakité A, Niaré SD, Guindo H, Akplogan A, Ibikounlé M, Boissier J, Dabo A. Ultrasound aspects and risk factors associated with urogenital schistosomiasis among primary school children in Mali. Infect Dis Poverty [Internet]. 2023 Apr 20 [cited 2025 Sep 16];12(1):40. Available from: https://idpjournal.biomedcentral.com/articles/10.1186/s40249-023-01071-6 https://doi.org/10.1186/s40249-023-01071-6
- Balogun JB, Adewale B, Balogun SU, Lawan A, Haladu IS, Dogara MM, Aminu AU, Caffrey CR, De Koning HP, Watanabe Y, Balogun EO. Prevalence and associated risk factors of urinary schistosomiasis among primary school pupils in the Jigawa and Zobiya communities of Jigawa state, Nigeria. Annals of Global Health [Internet]. 2022 Aug 16 [cited 2025 Sep 16];88(1):71. Available from: https://annalsofglobalhealth.org/articles/10.5334/aogh.3704/ https://doi.org/10.5334/aogh.3704
- Alidou S, Kamassa HE, Lack F, Ataba E, Fleming FM, Sossou E, Hemou M, Yakpa K, Tchalim M, Gnossike P, Vounatsou P, Pullan R, Gass K, Dorkenoo AM. Risk factors associated with urogenital schistosomiasis: a multilevel assessment approach using an Oversampling Schistosomiasis Survey (Sos) community-based, Plateaux region, Togo 2022. bmjph [Internet]. 2025 Jan [cited 2025 Sep 16];3(1):e001304. Available from: https://bmjpublichealth.bmj.com/lookup/doi/10.1136/bmjph-2024-001304 https://doi.org/10.1136/bmjph-2024-001304
- Joof E, Sanyang AM, Camara Y, Sey AP, Baldeh I, Jah SL, Ceesay SJ, Sambou SM, Sanyang S, Wade CM, Sanneh B. Prevalence and risk factors of schistosomiasis among primary school children in four selected regions of The Gambia. PLoS Negl Trop Dis [Internet]. 2021 May 11 [cited 2025 Sep 16];15(5):e0009380. Available from: https://dx.plos.org/10.1371/journal.pntd.0009380 https://doi.org/10.1371/journal.pntd.0009380
- Owusu G, Iddrisu AK, Antwi-Adjei M, Asare TA, Gyekyebea P, Opoku-Kusi R, Effah E, Tuekpe RM. Assessment of the prevalence and praziquantel effectiveness and risk factors of urogenital schistosomiasis among school-aged children in pru east, Ghana. Sci Rep [Internet]. 2025 Jun 3 [cited 2025 Sep 16];15(1):19376. Available from: https://www.nature.com/articles/s41598-025-96653-9 https://doi.org/10.1038/s41598-025-96653-9
- Essien-Baidoo S, Essuman MA, Adarkwa-Yiadom B, Adarkwa D, Owusu AA, Amponsah SB. Urinogenital schistosomiasis knowledge, attitude, practices, and its clinical correlates among communities along water bodies in the Kwahu Afram Plains North District, Ghana. PLoS Negl Trop Dis [Internet]. 2023 Aug 16 [cited 2025 Sep 16];17(8):e0011513. Available from: https://dx.plos.org/10.1371/journal.pntd.0011513 https://doi.org/10.1371/journal.pntd.0011513
- Wiegand RE, Secor WE, Fleming FM, French MD, King CH, Deol AK, Montgomery SP, Evans D, Utzinger J, Vounatsou P, De Vlas SJ. Associations between infection intensity categories and morbidity prevalence in school-age children are much stronger for Schistosoma haematobium than for S. mansoni. PLoS Negl Trop Dis [Internet]. 2021 May 25 [cited 2025 Sep 16];15(5):e0009444. Available from: https://dx.plos.org/10.1371/journal.pntd.0009444 https://doi.org/10.1371/journal.pntd.0009444
- Grolimund CM, Bärenbold O, Hatz CF, Vennervald BJ, Mayombana C, Mshinda H, Utzinger J, Vounatsou P. Infection intensity-dependent accuracy of reagent strip for the diagnosis of Schistosoma haematobium and estimation of treatment prevalence thresholds. PLoS Negl Trop Dis [Internet]. 2022 Apr 25 [cited 2025 Sep 16];16(4):e0010332. Available from: https://dx.plos.org/10.1371/journal.pntd.0010332 https://doi.org/10.1371/journal.pntd.0010332
- Degarege A, Animut A, Negash Y, Erko B. Performance of urine reagent strips in detecting the presence and estimating the prevalence and intensity of schistosoma haematobium infection. Microorganisms [Internet]. 2022 Oct 19 [cited 2025 Sep 16];10(10):2062. Available from: https://www.mdpi.com/2076-2607/10/10/2062 https://doi.org/10.3390/microorganisms10102062
- Mohammed H, Landeryou T, Chernet M, Liyew EF, Wulataw Y, Getachew B, Difabachew H, Phillips A, Maddren R, Ower A, Mekete K, Belay H, Endrias T, Anjulo U, Tasew G, Anderson R, Tollera G, Abate E. Comparing the accuracy of two diagnostic methods for detection of light Schistosoma haematobium infection in an elimination setting in Wolaita Zone, South Western Ethiopia. PLoS ONE [Internet]. 2022 Apr 29 [cited 2025 Sep 16];17(4):e0267378. Available from: https://dx.plos.org/10.1371/journal.pone.0267378 https://doi.org/10.1371/journal.pone.0267378
