Perspective | Open Access | Volume 8 (4): Article 84 | Published: 16 Oct 2025
Implementation of laboratory quality management system: A cornerstone for enhanced patient safety and clinical outcomes in Tanzania
Menu, Tables and Figures
On Google Scholar
Navigate this article
Figures
Keywords
- Laboratory
- Quality Management System
Vulstan James Shedura1, &, Kenan Kenan Malindisa2, Shaibu Jabiri Nanyanga2
1Department of Clinical Research, Training, and Consultancy, Southern Zone Referral Hospital, Mtwara, Tanzania, 2Department of Clinical Laboratory, Southern Zone Referral Hospital, Mtwara, Tanzania
&Corresponding author: Vulstan James Shedura, Department of Clinical Research, Training, and Consultancy, Southern Zone Referral Hospital, P.O. Box 272, Mtwara, Tanzania; Email: vulstanshedura@gmail.com ORCID: https://orcid.org/0000-0002-1939-2492
Received: 05 Jan 2025, Accepted: 15 Oct 2025, Published: 16 Oct 2025
Domain: Laboratory Capacity Development, Health Systems Strengthening
Keywords: Laboratory, Quality Management System
©Vulstan James Shedura et al. Journal of Interventional Epidemiology and Public Health (ISSN: 2664-2824). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Vulstan James Shedura et al., Implementation of laboratory quality management system: A cornerstone for enhanced patient safety and clinical outcomes in Tanzania. Journal of Interventional Epidemiology and Public Health. 2025;8(4):84. https://doi.org/10.37432/jieph-d-25-00011
Abstract
Laboratory Quality Management Systems (LQMS) is a structured framework that integrates policies, processes, and procedures to manage and improve the quality and reliability of laboratory services. The LQMS primarily concentrates on overseeing and ensuring quality within the laboratory’s operations. It encompasses the full testing lifecycle, including sample collection, handling, analysis, result reporting, and corrective actions to ensure valid testing results. It also manages documentation, audits, equipment calibration, personnel competence, and issues to minimize errors and increase reliability.
In Tanzania, the implementation of LQMS has been a progressive effort, guided by structured programs such as the Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA), the Strengthening Laboratory Management Toward Accreditation (SLMTA) program, and laboratory accreditation initiatives under ISO 15189. These efforts have contributed to a steady increase in the number of accredited laboratories and the scope of accredited tests, enhancing diagnostic services across different levels of healthcare.
Despite these advancements, LQMS implementation faces significant challenges, including inadequate infrastructure, financial constraints, staff shortages, and limited training for laboratory personnel. The absence of a national accreditation body compels laboratories to seek costly accreditation services from external agencies, further straining available resources. Additionally, challenges related to the sustainability of External Quality Assessment (EQA) programs, including logistical constraints and reliance on donor-funded vertical programs for Proficiency Testing (PT), hinder consistent quality assurance. To address these gaps, Tanzania must prioritize the establishment of an in-country accreditation body, expand EQA management centers, and secure sustainable financing for all PT programs.
Laboratories play a critical role in ensuring the quality of patient care and safety by providing accurate, timely, and reliable diagnostic information that guides clinical decision-making. Implementation of LQMS strengthens this mandate by enhancing diagnostic accuracy, reducing turnaround times, and promoting evidence-based treatment. Sustaining these gains requires continuous mentorship, investment in laboratory infrastructure, integration of Laboratory Information Management Systems, and government-led financing to ensure long-term healthcare quality and patient safety.
Perspective
Introduction
Laboratory medicine forms the backbone of evidence-based healthcare by providing critical diagnostic data that influences over 70% of clinical decisions [1]. Laboratory Quality Management Systems (LQMS) is a structured approach to managing laboratory operations that incorporates quality control, staff competency, equipment maintenance, and process standardization to meet international standards such as International Organization for Standardization (ISO 15189), titled “Medical Laboratories-Requirements for quality and competence [1, 2]. The principal goal of LQMS is to ensure accurate and reliable diagnostic services, reduce errors, and improve patient outcomes [1]. Despite its critical importance, laboratory services in low- and middle-income countries like Tanzania face significant systemic challenges that hinder their quality. These challenges include limited funding, insufficient infrastructure, and inadequate training for laboratory personnel [3, 4]. These barriers compromise the ability of laboratories to provide reliable results, which consequently compromises patient care and safety. Globally, adopting LQMS has consistently improved diagnostic accuracy, shortened turnaround times, and enhanced patient outcomes [5]. In Tanzania, supporting the implementation of LQMS is vital not only for improving routine diagnostic services but also for strengthening the healthcare system’s capacity to respond effectively to public health emergencies, including antimicrobial resistance (AMR) and emerging infectious diseases [3, 4].
Hospitals are central to healthcare delivery, often handling complex medical cases that require precise and timely diagnostic results. Implementing LQMS in hospitals ensures that laboratory processes are standardized, reducing errors and building confidence in the quality of laboratory-generated data. Furthermore, it aligns hospital laboratories with national and international accreditation standards, enhancing their credibility and operational efficiency [5]. By ensuring quality diagnostic results, hospitals can provide better clinical care and improve patient safety.
Additionally, LQMS promotes a culture of continuous quality improvement and compliance with national and international accreditation standards, thereby strengthening the overall health system. In this context, LQMS is not only a tool for laboratory improvement but also a cornerstone for advancing patient safety and improving clinical outcomes in hospitals.
Effect of quality laboratory services on patient safety and better treatment outcomes
Quality laboratory services are central to achieving patient safety and improving clinical outcomes because they form the evidentiary foundation of diagnosis, treatment, and disease monitoring. Globally, laboratory test results play a crucial role in guiding clinical decisions and shaping the overall quality of patient care [1] . A well-functioning LQMS ensures that each step of the diagnostic process, from sample collection, analysis, and interpretation to result reporting, is standardized, traceable, and accurate, thereby minimizing diagnostic errors that could lead to patient harm [2]. The mechanism by which quality laboratory services improve patient safety lies in the reduction of pre-analytical, analytical, and post-analytical errors, which collectively account for up to 60–70% of diagnostic mistakes [5]. Accurate and reliable results enable clinicians to initiate timely and appropriate treatment, optimize drug selection, reduce unnecessary prescriptions, and prevent adverse drug reactions. Conversely, poor-quality laboratory services may result in misdiagnosis, delayed treatment, inappropriate therapy, or prolonged hospitalization, all of which increase morbidity, mortality, and healthcare costs [6].
Empirical evidence from sub-Saharan Africa reinforces these outcomes. For instance, laboratories in Uganda, Kenya, and Tanzania implementing Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA) and Strengthening Laboratory Management Toward Accreditation (SLMTA) programs have reported a >30% reduction in diagnostic turnaround time and improved clinician trust through more accurate test results, leading to faster initiation of lifesaving treatments for HIV, TB, and malaria cases [7, 8]. In Ghana, strengthened LQMS frameworks reduced blood transfusion errors and prevented adverse reactions, while in Ethiopia, ISO 15189 accreditation enhanced bacteriology accuracy and reduced inappropriate antibiotic use[9, 10]. Conversely, weak or absent laboratory quality systems contribute to misdiagnosis, delayed or inappropriate therapy, and missed detection of AMR, resulting in prolonged illness, preventable deaths, and increased treatment costs. Poor-quality laboratories also drive AMR emergence by promoting empirical and irrational antibiotic prescribing, a challenge that remains critical in Africa[5, 10].
In the Tanzanian context, strengthening LQMS implementation directly contributes to the national agenda for patient safety and better outcomes by integrating quality assurance into clinical and public health laboratories. Facilities implementing ISO 15189 standards and participating in SLIPTA and SLMTA programs have demonstrated marked improvements in diagnostic accuracy, turnaround time, and clinician confidence in laboratory data [3, 4]. For instance, standardized internal quality control (IQC) and participation in External Quality Assessment (EQA) schemes promote the early detection of analytical errors and continuous improvement in test reliability. Moreover, a functional LQMS enhances infection prevention and control (IPC) by ensuring proper specimen handling, biosafety compliance, and traceability, which are critical elements for preventing nosocomial infections and laboratory-acquired hazards [1]. The adoption of quality indicators, such as error rates, turnaround time, and clinician satisfaction, also provides measurable metrics linking laboratory performance with patient outcomes, enabling evidence-based decision-making and accountability.
The synergy between LQMS and patient safety operates through a systems-based approach that fosters a culture of quality, risk mitigation, and continuous improvement. Laboratories adhering to quality standards provide clinicians with timely, accurate, and reproducible results, thereby supporting effective clinical decisions and rational therapeutics. This leads to improved patient safety through early disease detection, reduced diagnostic uncertainty, and optimized treatment outcomes. Conversely, patient safety feedback mechanisms drive laboratories to maintain high-quality performance and continuous compliance with standards. Thus, the implementation of LQMS represents not merely a technical intervention but a patient-centered, systemic quality assurance mechanism, making it a true cornerstone for enhancing patient safety and clinical outcomes in Tanzania.
Experiences of LQMS implementation in Tanzania
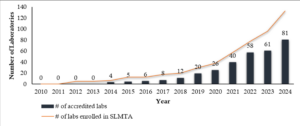
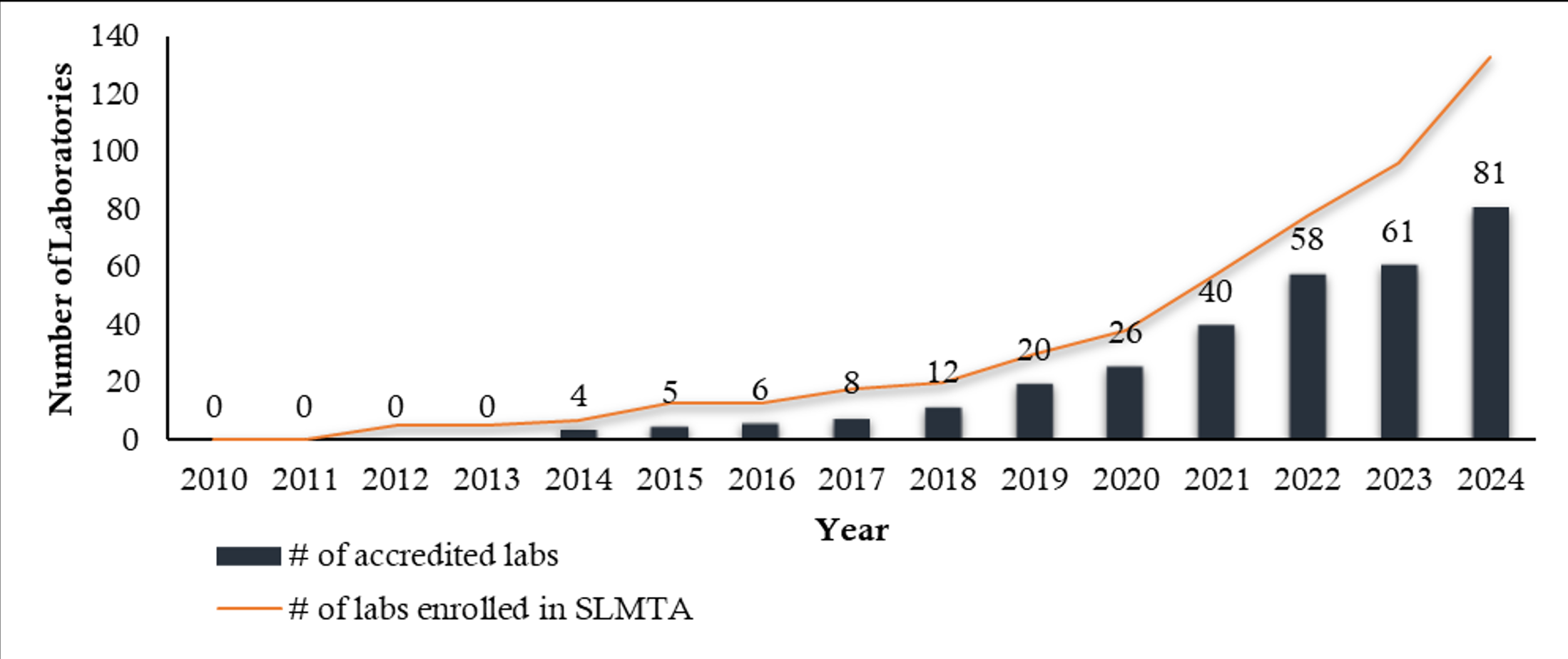
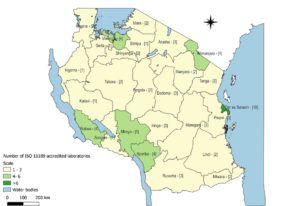
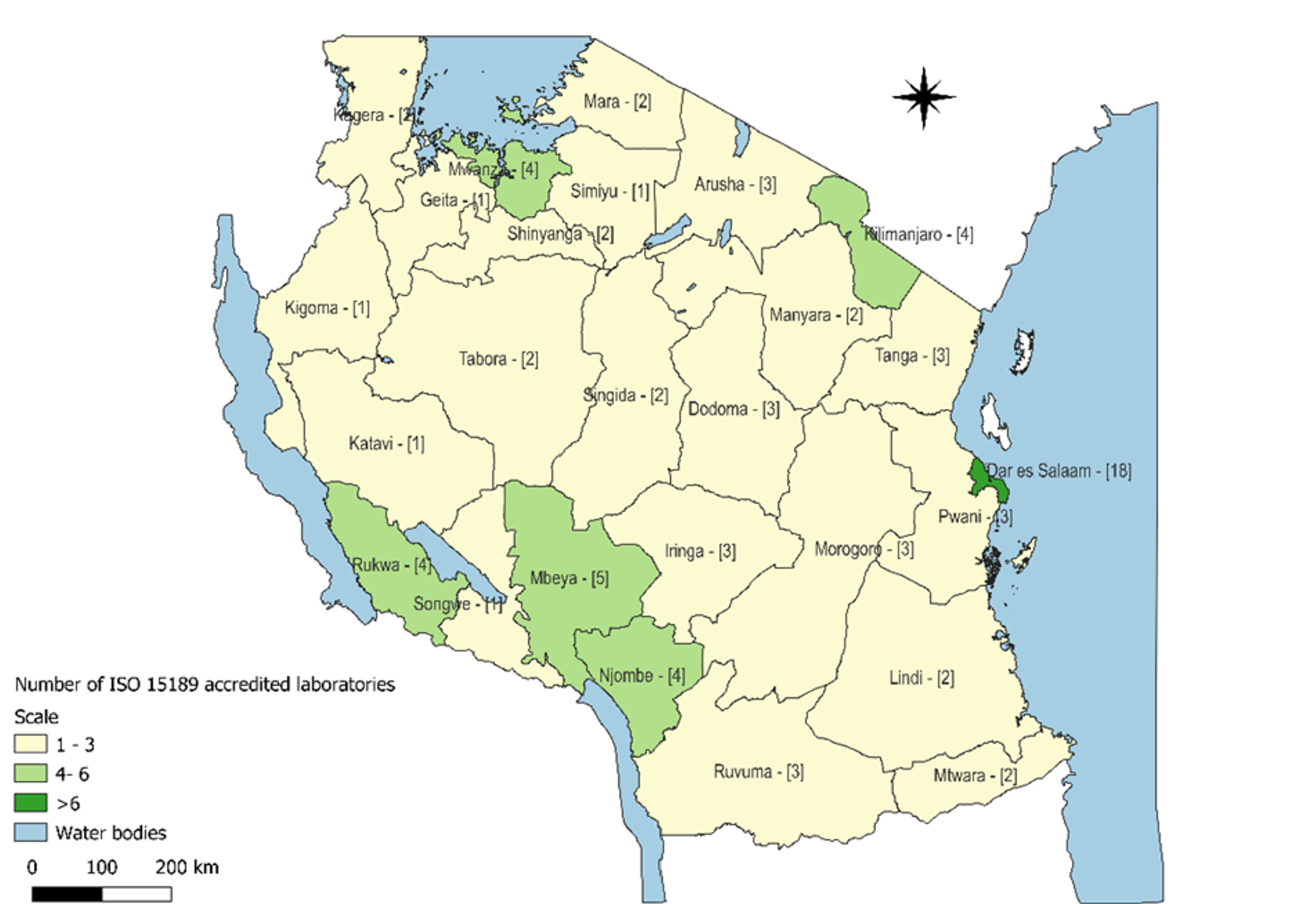
The implementation of LQMS in Tanzania has been a progressive effort aimed at enhancing patient safety and clinical outcomes [3]. The country has adopted structured programs such as the pre-SLMTA initiative, the SLMTA program, and laboratory accreditation efforts under ISO 15189 [4]. These initiatives have led to an increasing total number of accredited laboratories in each region (Figures 1 and 2) and accredited scopes yearly in Tanzania from 2010 to 2024, which have significantly contributed to improving laboratory services across different levels of the healthcare system (Figure 3).
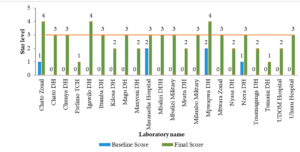
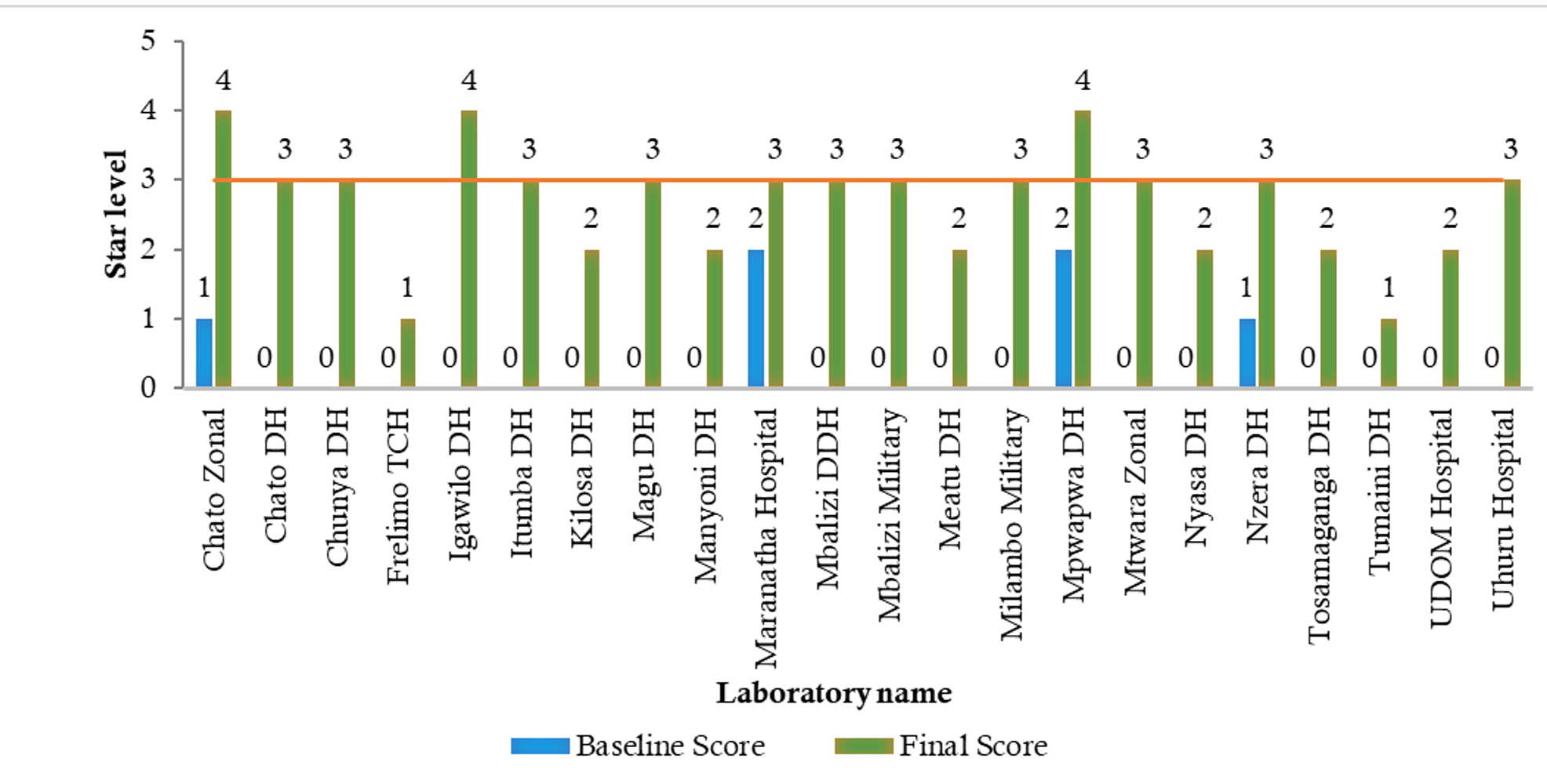
A key driver of QMS implementation has been the continuous training and mentorship of laboratory personnel[4]. The SLMTA program, which focuses on equipping laboratory staff with essential quality management skills, has led to notable improvements in performance. Regular mentorship exercises have played a critical role in guiding laboratories through the accreditation process, with more than 63.6% (14/22) of participating laboratories achieving good performance (3 stars and above) due to mentorship support (Figure 4).
However, challenges such as staff shortages, high turnover rates, and a lack of updated training for QMS mentors remain significant barriers to sustainability. To address these issues, there is a need to increase awareness, provide structured support for staff retention, and ensure timely payment of mentors during site visits.
Laboratory infrastructure in Tanzania varies widely, with some facilities facing significant challenges in equipment maintenance and availability. The lack of adequate support services, such as Information and Communication Technology (ICT) systems, remains a setback towards QMS implementation in Tanzania[4]. Laboratory Information Management Systems (LIMS) have been noted to have deficiencies in reporting capabilities, making it difficult to fully comply with ISO 15189:2022 requirements. Additionally, laboratory relocations, particularly in referral and district hospitals, have created disruptions in QMS implementation. Addressing these issues requires coordinated efforts, including increased investment in laboratory infrastructure, better ICT integration, and the development of contingency plans to ensure service continuity during facility relocations.
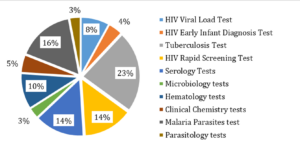
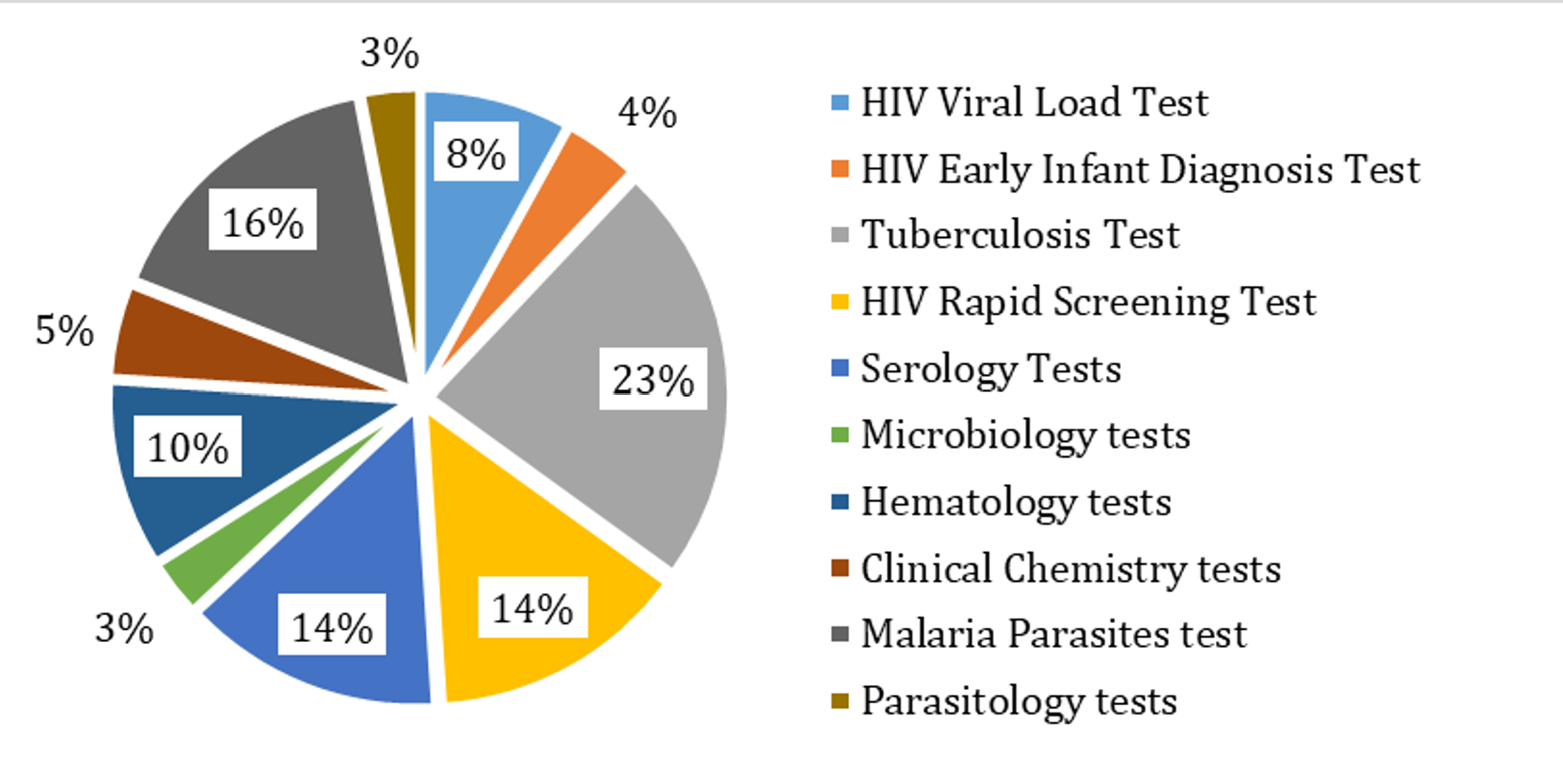
External Quality Assessment programs are integral to evaluating laboratory performance, identifying potential errors, and ensuring the reliability of patient test results[6]. In Tanzania, the External Testing Advisory Committee (ETAC) oversees the coordination of Proficiency Testing (PT) programs through major institutions, including the Central Tuberculosis Reference Laboratory (CTRL), the National Public Health Laboratory (NPHL), and the National Blood Transfusion Service (NBTS). These institutions provide PT panels covering a wide range of diagnostic tests, including tuberculosis (GeneXpert and microscopy), malaria rapid diagnostic testing, syphilis, hepatitis B virus, hematological tests, HIV rapid testing, and viral load monitoring. The implementation of these programs has strengthened the quality of laboratory services, though challenges such as inadequate funding, delayed accreditation processes, and logistical constraints in PT sample distribution persist. Addressing these gaps requires continued investment in local EQA capacity and enhanced coordination among stakeholders.
Sustaining QMS programs requires adequate funding, yet many laboratories struggle with financial constraints[4]. Currently, Tanzania lacks an in-country accreditation body, which forces laboratories to seek costly accreditation services from external agencies. The Ministry of Health (MOH) and partners have been urged to prioritize budget allocations for laboratory accreditation and equipment maintenance. Additionally, the establishment of a legal framework for an in-country accreditation body is essential to reduce costs and improve accessibility to accreditation services.
Despite these challenges, Tanzania has made significant progress in QMS implementation, with an increasing number of laboratories achieving accreditation and demonstrating improved service delivery (Figure 1). Strengthening staff training, infrastructure, and funding mechanisms will be critical in sustaining these gains and ensuring that laboratory services continue to support high-quality patient care.
The implementation of LQMS in Tanzania faces a range of challenges that hinder its progress and effectiveness. Limited financial resources and inadequate infrastructure remain persistent barriers, affecting the ability of laboratories to deliver consistent quality services [4]. The shortage of adequately trained laboratory personnel makes the problem worse, creating gaps in capacity and performance[4]. Resistance to change, both among clinical professionals and administrative staff, further obscures efforts to adopt new systems and practices. Additionally, inconsistent support from institutional and governmental levels results in uneven progress and sustainability of LQMS initiatives across different healthcare facilities[4].
However, the implementation of LQMS is a key strategy for enhancing patient safety and clinical outcomes, particularly in Tanzania, where health systems often face challenges such as resource constraints, staff shortages, and inadequate training [3, 4]. Evidence from studies in Ethiopia highlights the critical role of LQMS in ensuring the reliability and timeliness of laboratory results [6]. For example, the assessment of 89 health centers in Oromia revealed that while 20.2% of laboratories achieved improved service levels beyond star zero, significant gaps persisted in meeting quality benchmarks, with factors such as the availability of Standardized Standard Operating Procedures (SSOPs), quality plans, and specimen guidelines significantly influencing outcomes [6]. Similarly, a systematic review revealed that despite the introduction of LQMS in Ethiopian medical laboratories in 2009, the overall status of quality management practices remained limited due to issues such as inadequate management support, high workloads, and limited access to on-the-job training [10]. These findings show the necessity for strong technical and managerial support, capacity-building initiatives, and the integration of LQMS into national health strategies in Tanzania to achieve sustainable improvements in laboratory quality services and, consequently, better clinical outcomes.
Recommendations and way forward for improved LQMS in Tanzania
To enhance LQMS in Tanzania, several key recommendations and strategic actions should be prioritized. First, strengthening the implementation of the SLIPTA and the SLMTA programs is essential to ensure continuous quality improvement [3, 4]. Capacity building through regular training, mentorship, and supervision of laboratory personnel should be emphasized to enhance competency in quality management practices. Additionally, the government and stakeholders should invest in modern laboratory infrastructure, equipment calibration, and standardized reagents to ensure reliable and accurate testing.
A critical step forward is the establishment of a National Accreditation Body to oversee and streamline laboratory accreditation processes, reducing dependence on foreign accrediting institutions while ensuring sustainability and local ownership of quality standards [3, 4]. Furthermore, there is a need to expand EQA management centers across different regions to improve access, participation, and efficiency in quality monitoring of laboratory testing [5]. Strengthening EQA participation and enforcing compliance with international accreditation standards, such as ISO 15189, will further enhance laboratory performance.
Additionally, sustainable financing mechanisms should be established to support LQMS implementation at all healthcare levels[5]. This includes securing government funding for PT schemes instead of relying solely on donor-funded vertical programs, and ensuring the continuous participation of laboratories in EQA schemes[5]. Collaboration between the public and private sectors, along with continuous policy reviews and updates, will facilitate the integration of best practices in laboratory services, particularly in Tanzania[5]. Lastly, digital transformation through the adoption of LIMS should be expanded to improve data accuracy, reporting, and decision-making. By implementing these recommendations, Tanzania can achieve a more robust and efficient laboratory system, ultimately improving diagnostic services and patient care.
Conclusion
The implementation of the LQMS in Tanzania stands as a cornerstone for enhancing patient safety and improving clinical outcomes. By strengthening the accuracy, reliability, and timeliness of diagnostic services, LQMS ensures that patient management decisions are evidence-based and aligned with international quality standards. The progressive adoption of LQMS across Tanzanian laboratories from 2010 to 2024 demonstrates tangible improvements in diagnostic performance, staff competence, and overall service delivery. Despite persistent challenges, such as limited resources, infrastructure gaps, and workforce constraints, sustained collaboration among the Ministry of Health, healthcare institutions, and international partners offers a clear path forward. Prioritizing LQMS implementation will not only consolidate Tanzania’s healthcare quality framework but also safeguard patient safety, promote effective treatment outcomes, and advance the nation’s contribution to the global health quality and safety agenda.
Acknowledgements
The authors extend their sincere gratitude to the Ministry of Health, Department of Diagnostic Services, particularly the Medical Laboratory Services and Quality Assurance Section, for providing data and for their invaluable support in the preparation of this work. Additionally, we acknowledge the Southern Zone Referral Hospital for its technical support.
Authors´ contributions
Conceptualization: Vulstan James Shedura, Kenan Kenan Malindisa, Shaibu Jabiri Nanyanga; Data curation: Vulstan James Shedura, Kenan Kenan Malindisa; Formal analysis: Vulstan James Shedura; Methodology: Vulstan James Shedura; Resources: Shaibu Jabiri Nanyanga; Supervision: Shaibu Jabiri Nanyanga; Writing ±original draft: Vulstan James Shedura, Kenan Kenan Malindisa, Shaibu Jabiri Nanyanga; Writing ± review and editing: Vulstan James Shedura, Kenan Kenan Malindisa, Shaibu Jabiri Nanyanga.




References
- World Health Organization. Laboratory Quality Management System: Handbook. Geneva (Switzerland): World Health Organization; 2011 Jan 1 [cited 2025 Oct 15]. 271 p. Available from: https://www.who.int/publications/i/item/9789241548274
- Richardson H. Medical laboratories — Requirements for quality and competence: An ISO perspective. Vox Sanguinis [Internet]. 2002 Aug [cited 2025 Oct 16];83(s1):333–5. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1423-0410.2002.tb05329.x
- Ministry of Health (TZ). National Guidelines for Laboratory Quality Improvement. Dodoma (Tanzania): Ministry of Health; 2021 [cited 2025 Oct 15]. 153 p. Available from: https://www.moh.go.tz/storage/app/uploads/public/65c/095/5c2/65c0955c2b4a4121708873.pdf
- Beyanga M, Gerwing-Adima L, Jackson K, Majaliwa B, Shimba H, Ezekiel S, Massambu C, Majige D, Mwasegaka M, Mtotela W, Mateta P, Kasang C. Implementation of the laboratory quality management system (ISO 15189): experience from bugando medical centre clinical laboratory – mwanza, tanzania. African Journal of Laboratory Medicine [Internet]. 2018 Jul 31 [cited 2025 Oct 15];7(1). Available from: http://www.ajlmonline.org/index.php/AJLM/article/view/657 DOI: 10.4102/ajlm.v7i1.657
- Alemnji GA, Zeh C, Yao K, Fonjungo PN. Strengthening national health laboratories in sub-Saharan Africa: a decade of remarkable progress. Tropical Med Int Health [Internet]. 2014 Feb 10 [cited 2025 Oct 15];19(4):450–8. Available from: https://onlinelibrary.wiley.com/doi/10.1111/tmi.12269 DOI: 10.1111/tmi.12269
- Mulleta D, Jaleta F, Banti H, Bekele B, Abebe W, Tadesse H, Eshetu L, Zewdu A, Botore A, Tadesse L, Debela T. The impact of laboratory quality management system implementation on quality laboratory service delivery in health center laboratories of oromia region, ethiopia. PLMI [Internet]. 2021 Jul 8 [cited 2025 Oct 15];13:7–19. Available from: https://www.dovepress.com/the-impact-of-laboratory-quality-management-system-implementation-on-q-peer-reviewed-fulltext-article-PLMI DOI: 10.2147/PLMI.S314656
- Yao K, McKinney B, Murphy A, Rotz P, Wafula W, Sendagire H, Okui S, Nkengasong JN. Improving quality management systems of laboratories in developing countries. American Journal of Clinical Pathology [Internet]. 2010 Sep 1 [cited 2025 Oct 15];134(3):401–9. Available from: https://academic.oup.com/ajcp/article/134/3/401/1766287 DOI: 10.1309/AJCPNBBL53FWUIQJ
- Nkengasong JF, Yao K, Onyebujoh P. Laboratory medicine in low-income and middle-income countries: progress and challenges. The Lancet [Internet]. 2018 May 12 [cited 2025 Oct 15];391(10133):1873–5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673618303088 DOI: 10.1016/S0140-6736(18)30308-8
- Kumah A. The future of patient safety in Ghana: challenges and opportunities. Front Health Serv [Internet]. 2025 Jul 8 [cited 2025 Oct 15];5:1581468. Available from: https://www.frontiersin.org/articles/10.3389/frhs.2025.1581468/full DOI: 10.3389/frhs.2025.1581468
- Mesganaw B, Fenta A, Hibstu Z, Belew H, Misganaw K, Belayneh M. Medical Laboratories Quality Management and Challenges in Ethiopia: A Systematic Review. Pathology and Laboratory Medicine International [Internet]. 2023 Mar 2 [cited 2025 Oct 15];15:13-26. Available from: https://www.dovepress.com/medical-laboratories-quality-management-and-challenges-in-ethiopia-a-s-peer-reviewed-fulltext-article-PLMI DOI: 10.2147/PLMI.S395895
