Research | Open Access | Volume 8 (4): Article 87 | Published: 24 Oct 2025
Timeliness of surveillance during the Mpox outbreak in the Volta Region of Ghana: Analysis of Mpox surveillance data
Menu, Tables and Figures
Navigate this article
Tables
| Variable | Frequency [N = 22] | Percentage (%) |
|---|---|---|
| Mean age ± SD | 24.9 ± 16.5 | |
| Age (years) | ||
| < 20 | 11 | 50.0 |
| 20–29 | 6 | 27.3 |
| 30+ | 5 | 22.7 |
| Sex | ||
| Female | 8 | 36.4 |
| Male | 14 | 63.6 |
| Ethnicity | ||
| Ewe | 20 | 90.9 |
| Others | 2 | 9.1 |
| Occupation | ||
| Farmer | 2 | 9.1 |
| Nurse | 1 | 4.6 |
| Skilled person | 2 | 9.1 |
| Student | 12 | 54.5 |
| Trader | 3 | 13.6 |
| Unemployed | 2 | 9.1 |
| Place of Residence | ||
| Rural | 9 | 40.9 |
| Urban | 13 | 59.1 |
Table 1: Demographic characteristics of suspected mpox cases in Volta region, 2022
| Variable | Timely n (%) | Delayed n (%) | Median time-to-detect (IQR) in days |
|---|---|---|---|
| Age (years) | |||
| < 20 | 7 (63.6) | 4 (36.4) | 10 (9–14) |
| 20–29 | 3 (50.0) | 3 (50.0) | 9 (8–34) |
| 30+ | 4 (80.0) | 1 (20.0) | 21 (21–21) |
| Sex | |||
| Female | 6 (75.0) | 2 (25.0) | 8 (8–34) |
| Male | 8 (57.1) | 6 (42.9) | 10 (9–21) |
| Ethnicity | |||
| Ewe | 12 (60.0) | 8 (40.0) | 10 (9–21) |
| Others | 2 (100.0) | 0 (0.0) | – |
| Occupation | |||
| Non-student | 6 (60.0) | 4 (40.0) | 9 (9–21) |
| Student | 8 (66.7) | 4 (33.3) | 10 (8–14) |
| Place of Residence | |||
| Rural | 5 (55.6) | 4 (44.4) | 9 (8–34) |
| Urban | 9 (69.2) | 4 (30.8) | 14 (10–21) |
Table 2: Demographic characteristics and early mpox detection in Volta Region, 2022
| Variable | Early Mpox detection n (%) | Delayed n (%) | Crude hazard ratio HR (95% CI) |
|---|---|---|---|
| Age group | |||
| <20 | 7 (63.6) | 4 (36.4) | Ref. |
| 20–29 | 3 (50.0) | 3 (50.0) | 0.97 (0.35, 2.71) |
| 30+ | 4 (80.0) | 1 (20.0) | 1.38 (0.45, 4.15) |
| Sex | |||
| Female | 6 (75.0) | 2 (25.0) | Ref. |
| Male | 8 (57.1) | 6 (42.9) | 0.56 (0.23, 1.38) |
| Ethnicity | |||
| Ewe | 12 (60.0) | 8 (40.0) | Ref. |
| Others | 2 (100) | 0 (0.0) | 3.08 (0.62, 15.26) |
| Occupation | |||
| Non-student | 1 (50.0) | 4 (40.0) | Ref. |
| Student | 8 (66.7) | 4 (33.3) | 1.34 (0.29, 6.33) |
| Place of Residence | |||
| Rural | 5 (55.7) | 4 (44.4) | Ref. |
| Urban | 9 (69.2) | 4 (30.8) | 1.56 (0.64, 3.8) |
Table 3: Factors associated with early mpox detection in Volta Region, 2022
Figures


Keywords
- Mpox
- Timeliness
- Outbreaks
- Ghana
Samuel Adolf Bosoka1,2, Joseph Asamoah Frimpong3,&, Joseph Yaw Jerela1,2, Dora Dadzie3, Dennis Odai Laryea4, Franklin Asideu-Bekoe5, Kwasi Senanu Djokoto1
1Volta Regional Health Directorate, Ghana Health Service, Ho, Ghana, 2Department of Epidemiology and Biostatistics, Fred N. Binka School of Public Health, University of Health and Allied Sciences, Ho, Ghana, 3Ghana Field Epidemiology and Laboratory Training Programme, University of Ghana, Accra, Ghana, 4Disease Surveillance Department, Ghana Health Service, Accra, Ghana, 5Public Health Division, Ghana Health Service, Accra, Ghana
&Corresponding author: Joseph Asamoah Frimpong, Ghana Field Epidemiology and Laboratory Training Programme, University of Ghana, Accra, Ghana, Email: asamoah.frimpong@gmail.com, ORCID: https://orcid.org/0000-0002-4758-0589
Received: 21 May 2025, Accepted: 24 Oct 2025, Published: 24 Oct 2025
Domain: Field Epidemiology, Outbreak Investigation
Keywords: Mpox, Timeliness, Outbreaks, Ghana
©Samuel Adolf Bosoka et al. Journal of Interventional Epidemiology and Public Health (ISSN: 2664-2824). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Samuel Adolf Bosoka et al., Timeliness of surveillance during the Mpox outbreak in the Volta Region of Ghana: Analysis of Mpox surveillance data. Journal of Interventional Epidemiology and Public Health. 2025;8(4):87. https://doi.org/10.37432/jieph-d-25-00128
Abstract
Introduction: Early detection, notification, and response to infectious disease outbreaks are crucial to prevent localized incidents from escalating into pandemics. Volta region of Ghana reported suspected cases of Mpox in 2022. We estimated the median time and the rate of timely detection, notification, and response to Mpox outbreaks in the region.
Methods: Secondary analysis of Mpox surveillance data in Volta Region from May 2022 to September 2022 was conducted. The 7-1-7 timeliness metrics were used to define timeliness of outbreak detection, notification, and response. Median timeliness was estimated with the Kaplan-Meier survival estimator. Cox’s proportional hazard model was used to identify factors associated with early Mpox detection. Significance level was considered at p < 0.05.
Results: Twenty-two suspected Mpox cases with a mean age of 24.9 ± 16.5 years were analysed. Males accounted for 63.6% (14/22). The median time from symptom onset to detection was 10 days [interquartile range (IQR): 9–21], with 63.6% of cases detected on time. The median time to case notification was 2 days (IQR: 2–4), with 90.9% of cases notified on time. Only 36.4% of cases were confirmed promptly, with median time to laboratory confirmation of 14 days (IQR: 9–24).
Conclusion: The surveillance system for Mpox in the Volta region of Ghana was generally not timely though it demonstrated commendable speed in case notification. However, the system lagged in detection and response, largely due to structural and logistic challenges. These challenges need improvement for effective response to Mpox and other public health threats in the region.
Introduction
Early detection, reporting, and response to infectious disease outbreaks are crucial to prevent localized incidents from escalating into global pandemics [1,2]. The swift response during the Marburg outbreak in Ghana [3] and the Ebola outbreak in Nigeria [4] exemplifies how rapid response can curb pandemic progression. The unfortunate delayed confirmation of Ebola in Guinea allowed the virus to spread to Liberia and Sierra Leone, resulting in severe consequences for health, economies, and the population [5]. These instances underscore the paramount importance of early detection, notification, and response in averting catastrophic outcomes.
The recent introduction of the 7-1-7 target presents a golden opportunity for countries to effectively evaluate the timeliness of surveillance systems at national or regional levels. The 7-1-7 is a global target that requires that every suspected outbreak is identified within seven days of emergence, reported to public health authorities within one day, and effectively responded to within 7 days of notification [6].
Studies have used timeliness as an attribute of surveillance systems to evaluate the performance of surveillance of different infectious disease systems [7–11]. In the African Region over three years from 2017 to 2019, Impouma and colleagues observed that the overall median time for detecting an outbreak was 8 days (IQR 2 to 28 days), 3 days (IQR 0 to 9 days) for notification, and 77 days (IQR 33 to 165 days) for initiation of response measures [12].
Limited studies have evaluated infectious disease surveillance systems through the lens of the time required to identify, report, and respond to an outbreak or unusual disease occurrence in the Volta Region. We estimated the median time and the rate of early detection, notification, and response to suspected mpox outbreaks within the surveillance system of the Volta region in Ghana.
Methods
Study Design and Population
We undertook a secondary analysis of mpox surveillance data in the Volta Region of Ghana from May 2022 to September 2022. We used timeliness as an attribute of the surveillance system to evaluate the performance of the mpox surveillance system. Timeliness for mpox detection, notification, and response was estimated compared to the 7-1-7 targets to evaluate the performance of the mpox surveillance system.
Study Site Description
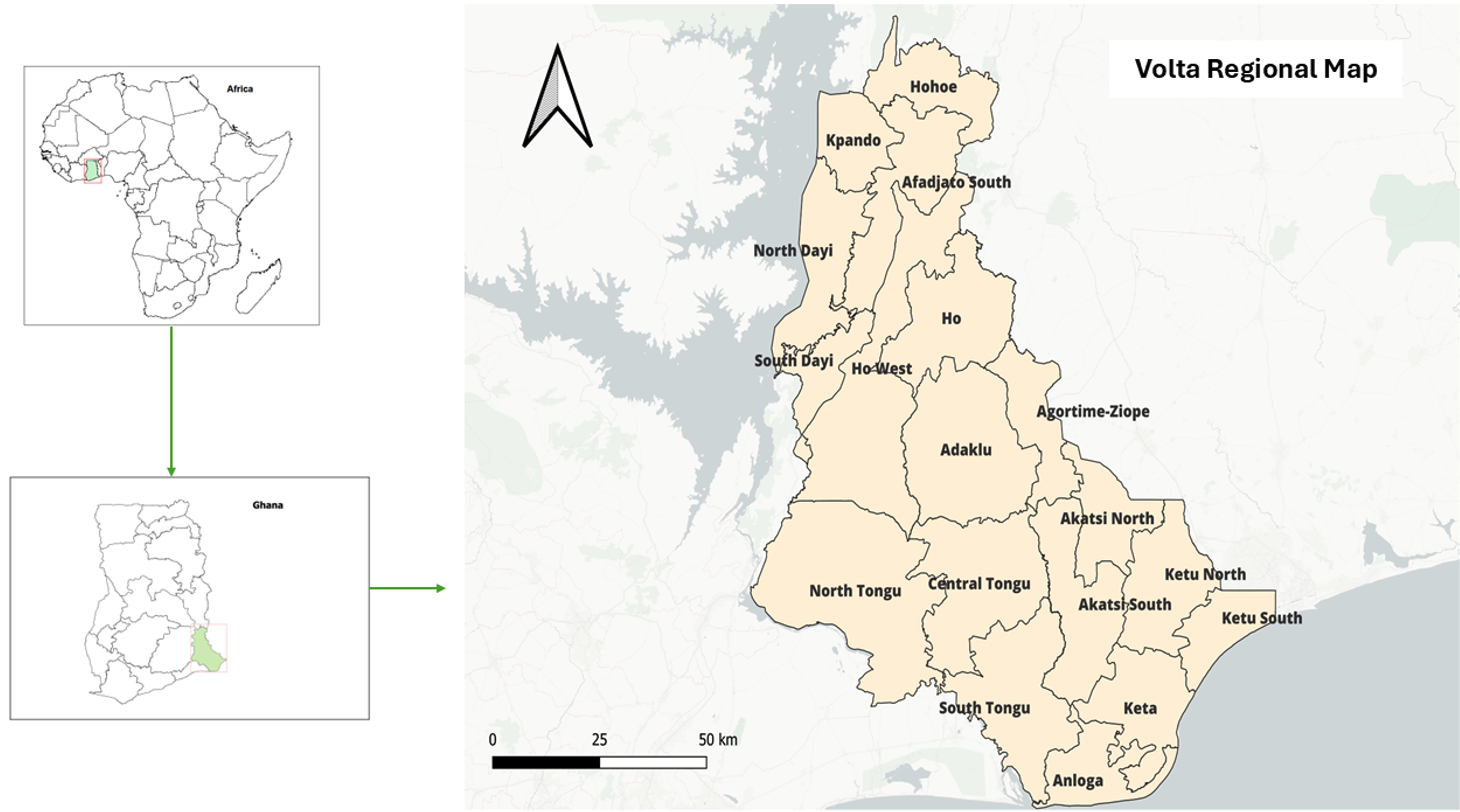
The study was conducted in the Volta Region, which is one of the sixteen administrative regions of Ghana (Figure 1). The region is located in the Eastern part of Ghana and shares borders with Togo. The region has a diverse landscape ranging from tropical rainforests to coastal plains, providing an ideal environment for a variety of animal species, including rodents, bats, and non-human primates that can serve as potential reservoirs for the mpox virus. The region occupies a surface area of about 20,570 square kilometres, and it is divided into 18 administrative districts [13]. The Ewe people are the native and largest ethnic group, constituting about 68.5% of the population.
Based on the 2021 National Population and Housing Census, the population of the region in the year under review was 2,033,754, with an annual average growth rate of 2.4% [13]. The region has a total of 732 health facilities, which comprise: 482 CHPS, 45 Clinics, 30 hospitals, 156 health centres, 14 maternity homes, 4 polyclinics, and a teaching hospital.
Outbreak of mpox in Volta
In August 2022, the Volta Region recorded its first confirmed cases of Mpox. The index case was a 39-year-old mechanic employed at a public university in the region. He first sought care at Ho Teaching Hospital on August 10, 2022, after experiencing fever, headache, body pain, sore throat, cough, fatigue, and a generalized rash. Prior to visiting the hospital, he had confided in a nurse friend, who encouraged him to consult a public health practitioner at the hospital’s Public Health Unit. His rash started on August 7 and had rapidly spread across his body at the time of clinical examination. During the assessment, the patient mentioned that his daughter had developed a similar rash without fever two days after his symptoms began. Based on this information, the municipal rapid response team promptly located the daughter for evaluation. She also presented with a widespread rash consistent with Mpox. They were isolated, and lesion swabs were collected and sent to the Noguchi Memorial Institute for Medical Research (NMIMR) for laboratory testing. Polymerase chain reaction (PCR) tests confirmed the presence of monkeypox virus DNA in both the father and daughter.
Data collection
We used the mpox line-list from the Volta regional surveillance unit to obtain epidemiological, clinical, and laboratory information of mpox cases from May 2022 to September 2022. Data was compared with case-based forms and data captured in the Surveillance Outbreak and Response Management Analysis System (SORMAS) to ensure good data quality. A suspected case was any person presenting with a history of sudden onset of fever, followed by a vesiculopustular rash occurring mostly on the face, palms, and soles of the feet.
Demographic data such as age, sex, and occupation, as well as clinical information such as clinical signs and symptoms of cases, were extracted. Time-to-event variables such as date case reported at the facility, date of onset of symptoms, date sample was taken, date laboratory sample was received, date feedback on laboratory results was reported to the DHD, and date of start of contact tracing were extracted from the SORMAS.
Operational definition and Measurement
Detection was measured as the duration between the date of symptom onset and the first date of reporting to a health facility. When the duration was ≤ 7 days, we describe the surveillance system as timely in mpox detection. For notification, we measured the duration between the date the case was first seen at the facility and the date the case was reported to public health authorities at the district level. The notification was described as timely when the interval was within one day. Response was measured as the interval between the date of notification, and the date laboratory feedback was received. The response was considered timely if the duration was within 7 days.
Data management and analysis
Time intervals between each date were measured in days. The cleaned dataset was imported into Stata version 17.0 for analysis. Continuous variables were reported as means with their respective standard deviations, while categorical variables were reported as proportions. We used the Kaplan-Meier survival estimator to estimate the median time to detect, notify, and respond to mpox. The interquartile range (IQR) was also reported. The Kaplan-Meier survival estimator was used to construct survival curves that displayed the timeliness of mpox case detection, notification, and response. QGIS was used to draw a choropleth map of timely case detection, notification, and respond.
We then used Cox’s proportional hazard model to estimate the hazard ratios (HR) with 95% confidence intervals (95% CI) to identify the factors associated with early mpox case detection. Variables were considered independent predictors of early case detection when p < 0.05.
Ethical Considerations
Administrative approval was sought from the Volta Regional Health Directorate. Confidentiality was ensured by removing all personal identifying information. This work was conducted as part of a routine performance evaluation by the Ghana Health Service and the Ghana Field Epidemiology and Laboratory Training Program (GFELTP) under the Ghana Public Health Act 851[14], hence no need to seek institutional ethical approval.
Results
Demographic characteristics of suspected mpox cases
The number of suspected mpox cases was 22, out of which 2 (9.1%) were confirmed by real-time polymerase chain reaction (RT-PCR). Males 14 (63.6%) were more affected than females. Half of the confirmed Mpox cases, 11 (50.0%), were among individuals aged less than 20 years. The overall mean age was 24 ± 16.5 years. The Ewe ethnic group was the most affected, 20 (90.9%). More than half (59.1%) of the outbreaks occurred in urban settings, and students were most affected (54.5%) (Table 1).
Timeliness of mpox case detection, notification, and response
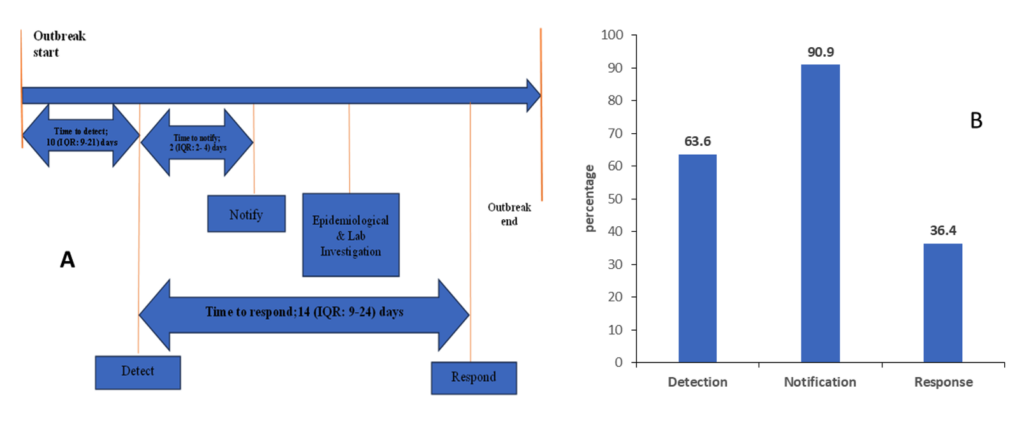
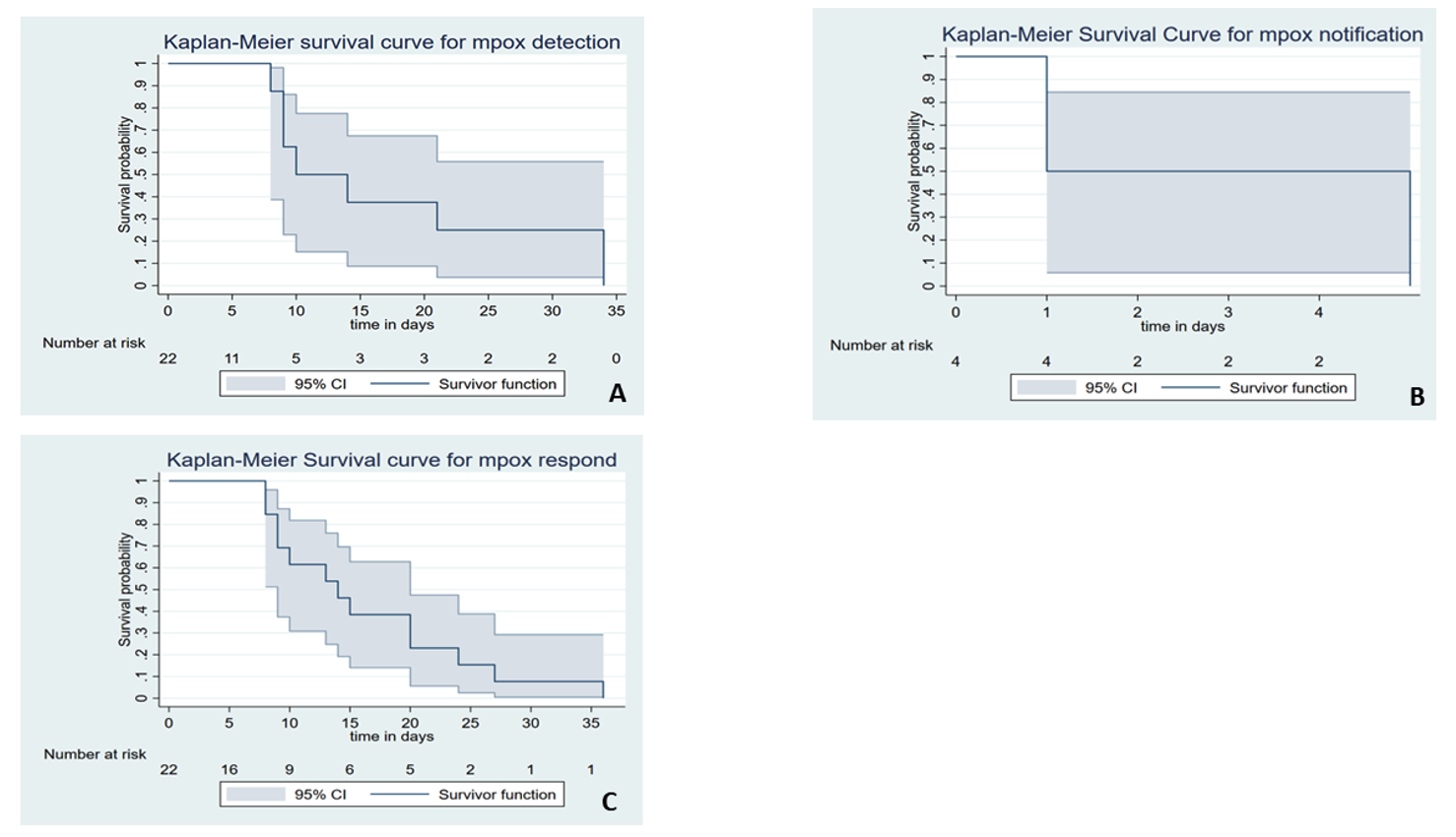
Of the 22 suspected mpox cases that were involved in the analysis, we found that the median time from symptom onset to detection was 10 days [interquartile rage (IQR): 9–21], with 63.6% (14/22) of cases detected in a timely manner. The median time to case notification was 2 days (IQR: 2–4), with 90.9% of cases notified on time. In contrast, only 36.4% of cases were confirmed promptly, with a median time from case notification to laboratory confirmation was 14 days (IQR: 9–24) (Figure 2).
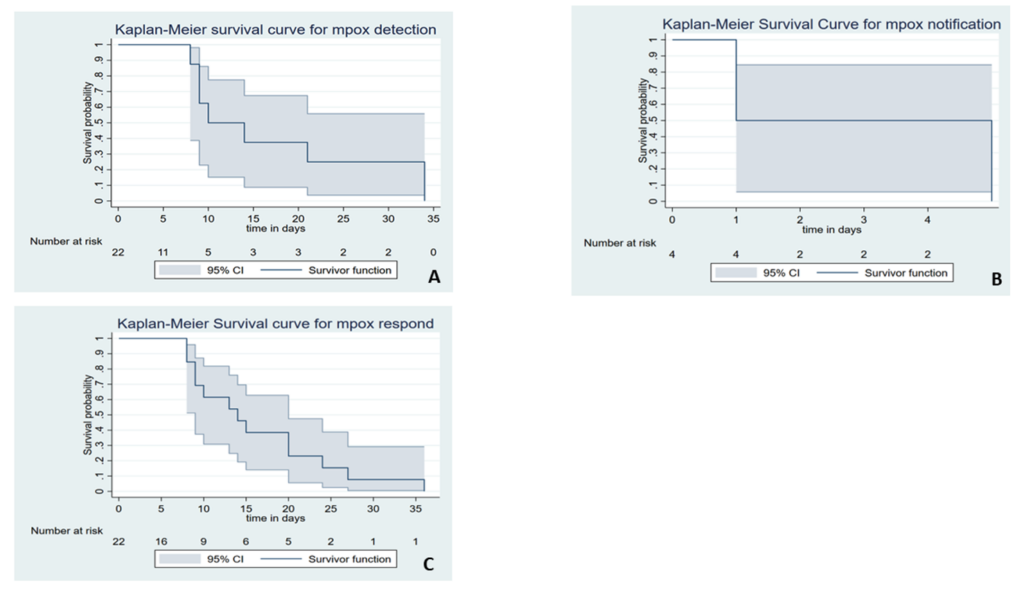
Figure 3 depicts the cumulative probability of timely detection, notification, and response by the surveillance system and its confidence intervals. The results show that the survival probability of early detection of mpox outbreak for at least 8 days was 88% [0.88 (95%CI; 0.39, 0.98)]. On the second day, 50% of the suspected outbreaks were notified to public health authorities [0.5 (95%CI; 0.01, 0.91)], and the system was able to timely confirm 86% [0.86 (95%CI; 0.54, 0.96)] of all the suspected mpox.
Demographic characteristics and early case detection of mpox
The median time to detection among case-patients aged less than 20 years and those aged 30 years and above was 10 (IQRange: 9-14) and 21 (IQRange: 21-21) days, respectively. Timely detection was higher among case-patients aged 30 years and above (80%) than among those less than 20 years (63.6%). The median time to detection for females was 8 (IQRange: 8-34) days, and the rate of timely detection by the surveillance system was higher among females (75.0% ) than in males (57.1%) Table 2.
Geospatial mapping of early mpox detection rate
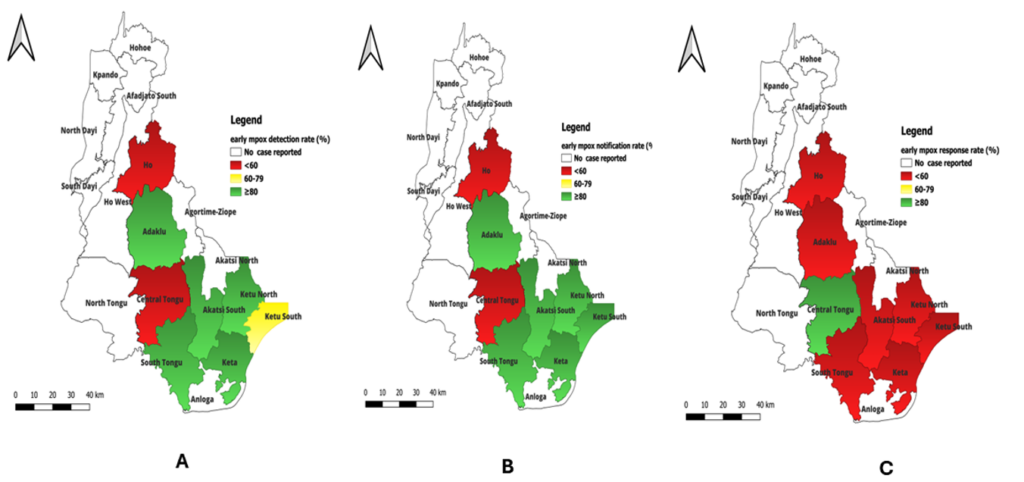
The results show that of the nine districts that recorded suspected mpox outbreaks, the rate of detection was less than 60% in Ho municipality and Central Tongu districts. Regarding Mpox notification, Ho municipality was the least performing district with less than 60% early notification rate. We observed that the rate of early response was less than 60% in all the districts except Central Tongu (Figure 4).
Factors associated with early mpox case detection
We found that age, sex, ethnicity, occupation, and place of residence were not statistically associated with early case detection. However, the risk of early case detection was 1.38 times higher among case-patients who were aged 30 years and above compared to those aged less than 20 years [crude hazard ratio cHR=1.38 (95% CI; 0.45, 4.15)]. Male case-patients were 44% less likely to experience early case detection as compared to female case-patients [crude hazard ratio cHR=0.56 (0.23, 1.38). Risk of early case detection was 1.34 times higher among students as compared to non-students [crude hazard ratio cHR=1.34 (0.29, 6.33)] (Table 3).
Discussion
The escalating global incidence of human mpox cases illustrates the importance of early detection, prevention, management, and timely response from health systems. Our study assessed the median time and the rate of rapid case detection, notification, and public health respond to human mpox within the surveillance system of the Volta Region in Ghana.
Our findings suggest that the surveillance system in the Volta Region was not timely in mpox detection. The median time to outbreak detection was higher than the national target of 3 days as recommended by the technical guidelines for integrated disease surveillance and response [15]. The performance was much higher than the 7-1-7 global target of seven days [6]. It was also higher than the 8 (IQR 2–28) days reported in a recent evaluation of outbreak response in the African Region [12]. The median time was also lower than the 12 days reported in Canada [10] and the 13.5 days reported by Chan and colleagues [11]. Our findings were similar to a previous report in Greater Accra, where it took 10 days between symptom onset and case detection for influenza-like illnesses at facilities [16].
The better performance of mpox surveillance in case detection as compared to the other epidemic prone diseases may be due to the fact that Mpox has a well-defined clinical presentation, including a distinctive rash that often prompts affected individuals to seek care. Additionally, the disease has a relatively long incubation period (typically 7–14 days) and does not usually involve asymptomatic or presymptomatic transmission. This allows for a clearer window of opportunity for detection before onward transmission occurs. These factors combined with heightened global awareness due to WHO’s emergency declaration may have enhanced detection efforts and accelerated surveillance system responses[1].
Our study revealed that the median time and the rate of mpox detection were better in females than in males. This finding might be due to the differences in the health-seeking behaviour of men as compared to women. Literature explains that women are more likely to access healthcare services as compared to men [17,18]. Outbreak detection is dependent on the health-seeking behaviour of patients [19]. Women are better off reporting illness at health facilities than men. A recent study in the Volta Region found that a higher proportion of women reported to a health facility after the onset of fever as compared to men [20]. Also, the likelihood of early case detection was higher among individuals aged 30 years and above compared to those under 20 years. This finding may be explained by the fact that older adults often have greater health literacy, which enables them to recognize symptoms of Mpox more readily and seek medical care in a timely manner. In contrast, younger individuals may have limited awareness of the disease or may mistake the symptoms such as rashes for less serious conditions like chickenpox, leading to delays in seeking care. We found that the median time for mpox case notification was relatively higher when compared to the 7-1-7 target, which recommends that notification of epidemic prone disease to public health authorities should be within a day [6]. The median time for case notification in our study was lower than the 3 (IQR 0–9) days reported by [12]. Regarding the rate of timely notification, our findings suggest that in every ten suspected mpox cases, nine were timely notified by the surveillance system in the Volta region. This implies that almost all suspected mpox cases in the Volta Region were timely notified to public health authorities for effective and rapid control. Although notification systems for infectious diseases are country-specific and therefore, difficult to compare [21], our finding was relatively higher than the 66.2% reported in Benin [22].
Our study reveals a concerning gap in Mpox response in the Volta Region. Fewer than half of the cases received a timely response. The median time from reporting to laboratory confirmation was twice the global target of 7 days [6]. These delays slow contact tracing, postpone case isolation, and reduce timely risk communication. They also increase the risk of sustained community transmission and weaken trust in surveillance data for decision-making.
Several factors explain these delays. Many districts face challenges transporting samples for early confirmation. Inadequate funding limits resources for surveillance, especially for moving samples to public health reference laboratories. The Noguchi Memorial Institute for Medical Research (NMIMR) in Accra confirms Mpox cases for the country. However, it is 138 km from the Volta regional capital, Ho. Because of the cost and distance, facilities often wait until several samples accumulate before sending them. This practice contributes to long delays in confirmation and weakens the effectiveness of outbreak response. Another challenge is limited use of the Surveillance Outbreak and Response Management Analysis System (SORMAS). Many facility laboratory officers do not use SORMAS to register and submit samples early. These further delays confirmation.
Despite these challenges, the median response time in our study was shorter than reported by Galanis and colleagues [10]. Strengthening laboratory capacity, improving sample transport, ensuring wider use of SORMAS, and addressing funding gaps are urgent priorities. These measures are critical to enhance early detection, speed up response, and align Ghana’s Mpox preparedness with global standards.
The discrepancies in the median durations for mpox detection, notification, and response can be attributed to variations in the statistical methodologies employed and the distinct infectious disease surveillance systems under evaluation. Galanis and colleagues utilized arithmetic mean and median calculations to estimate the time for notification and response. However, these methods do not adequately account for the event aspect of the data. In contrast, our analysis employed survival or duration analysis using the Kaplan-Meier estimate, which is particularly well-suited for analyzing durations and event data. Time-to-event (TTE) data are unique due to the dual nature of the outcome of interest: not only whether an event occurred, but also when it occurred. Conventional approaches such as logistic and linear regression are not suited to be able to include both the event and time aspects as the outcome in the model. Furthermore, these traditional regression methods lack the capacity to effectively manage censoring, a specialised form of missing data encountered in time-to-event analyses when subjects do not experience the event of interest during the follow-up period [23].
The findings underscore the need to expand laboratory capacity and improve specimen transport systems to reduce turnaround times, not only for Mpox but also for other epidemic-prone diseases in Ghana. It also highlighted the importance of investing in community awareness and engagement to promote earlier health-seeking behaviour. Optimising digital platforms such as SORMAS for faster, more reliable data transmission could enhance coordination across districts and regions. Addressing these systemic gaps will improve Ghana’s ability to detect, notify, and confirm cases more swiftly. Beyond strengthening Mpox surveillance, such improvements will build a more resilient health security system, better equipped to manage future outbreaks.
Limitation
Firstly, it was a retrospective review of mpox surveillance data, and the dataset was not originally gathered for assessing timeliness metrics, potentially limiting the depth of analysis. Secondly, the study focused on a single outbreak event, limiting the generalizability of the surveillance system’s overall performance. Third, our timeliness assessment was confined to the first three components of the 7-1-7 response target (response initiation, epidemiological investigation, and laboratory confirmation). Laboratory confirmation was used as the primary measure because the dataset lacked information on the remaining four components: medical treatment, countermeasures, communication and community engagement, and response coordination. As a result, our findings may not fully capture the wider scope of early response activities. Lastly, most outbreaks were suspected rather than confirmed, potentially influencing the urgency of response. It is also important to consider the context that this outbreak occurred during a global Mpox emergency, drawing significant international attention, including a WHO public health emergency declaration, which may have prompted faster-than-usual response efforts, such as rapid diagnostic setup. Despite these limitations, the study demonstrates that outbreak metrics can be collected and measured using the 7-1-7 approach to identify systemic gaps in the surveillance system.
Conclusion
The Volta Region’s mpox surveillance system demonstrates commendable speed in case notification but continues to lag in detection and response, largely due to structural and logistical challenges. Addressing these bottlenecks, particularly limited laboratory access, weak digital data flows, and delayed healthcare-seeking behaviour, is critical to achieving global response standards. Targeted investments in improving laboratory connectivity, strengthening the use of SORMAS for real-time reporting, and enhancing community awareness could significantly improve system performance. Lessons from these findings can guide preparedness and surveillance strengthening in other regions of Ghana and inform responses to future outbreaks.
What is already known about the topic
- WHO African Region has applied survival analysis in evaluation of timeliness of surveillance.
- Timeliness definition is not standardized and therefore difficult to compare studies.
- The 7-1-7 target presents a golden opportunity for countries to effectively evaluate the timeliness of surveillance systems at national or regional levels.
What this study adds
- Using 7-1-7 targets for assessing surveillance systems in early detection, notification, and outbreak response provides a standardized approach to identify areas where performance gaps exist and require improvement.
- The duration from reporting, notification to laboratory confirmation needs to be improved for an effective response to public health threats.
Authors´ contributions
SAB, and JYJ conceived of the idea, with JAF and DD, contributing to the evaluation design. SAB, KSD, JAF and JYJ supported acquiring, managing, and interpreting the data. SAB, JYJ, and JAF and DD supported the analysis. The manuscript was prepared by SAB and JAF, and all authors contributed to writing and revision of the manuscript. All authors approved the final version and submission of the manuscript for publication.
| Variable | Frequency [N = 22] | Percentage (%) |
|---|---|---|
| Mean age ± SD | 24.9 ± 16.5 | |
| Age (years) | ||
| < 20 | 11 | 50.0 |
| 20–29 | 6 | 27.3 |
| 30+ | 5 | 22.7 |
| Sex | ||
| Female | 8 | 36.4 |
| Male | 14 | 63.6 |
| Ethnicity | ||
| Ewe | 20 | 90.9 |
| Others | 2 | 9.1 |
| Occupation | ||
| Farmer | 2 | 9.1 |
| Nurse | 1 | 4.6 |
| Skilled person | 2 | 9.1 |
| Student | 12 | 54.5 |
| Trader | 3 | 13.6 |
| Unemployed | 2 | 9.1 |
| Place of Residence | ||
| Rural | 9 | 40.9 |
| Urban | 13 | 59.1 |
| Variable | Timely n (%) | Delayed n (%) | Median time-to-detect (IQR) in days |
|---|---|---|---|
| Age (years) | |||
| < 20 | 7 (63.6) | 4 (36.4) | 10 (9–14) |
| 20–29 | 3 (50.0) | 3 (50.0) | 9 (8–34) |
| 30+ | 4 (80.0) | 1 (20.0) | 21 (21–21) |
| Sex | |||
| Female | 6 (75.0) | 2 (25.0) | 8 (8–34) |
| Male | 8 (57.1) | 6 (42.9) | 10 (9–21) |
| Ethnicity | |||
| Ewe | 12 (60.0) | 8 (40.0) | 10 (9–21) |
| Others | 2 (100.0) | 0 (0.0) | – |
| Occupation | |||
| Non-student | 6 (60.0) | 4 (40.0) | 9 (9–21) |
| Student | 8 (66.7) | 4 (33.3) | 10 (8–14) |
| Place of Residence | |||
| Rural | 5 (55.6) | 4 (44.4) | 9 (8–34) |
| Urban | 9 (69.2) | 4 (30.8) | 14 (10–21) |
| Variable | Early Mpox detection n (%) | Delayed n (%) | Crude hazard ratio HR (95% CI) |
|---|---|---|---|
| Age group | |||
| <20 | 7 (63.6) | 4 (36.4) | Ref. |
| 20–29 | 3 (50.0) | 3 (50.0) | 0.97 (0.35, 2.71) |
| 30+ | 4 (80.0) | 1 (20.0) | 1.38 (0.45, 4.15) |
| Sex | |||
| Female | 6 (75.0) | 2 (25.0) | Ref. |
| Male | 8 (57.1) | 6 (42.9) | 0.56 (0.23, 1.38) |
| Ethnicity | |||
| Ewe | 12 (60.0) | 8 (40.0) | Ref. |
| Others | 2 (100) | 0 (0.0) | 3.08 (0.62, 15.26) |
| Occupation | |||
| Non-student | 1 (50.0) | 4 (40.0) | Ref. |
| Student | 8 (66.7) | 4 (33.3) | 1.34 (0.29, 6.33) |
| Place of Residence | |||
| Rural | 5 (55.7) | 4 (44.4) | Ref. |
| Urban | 9 (69.2) | 4 (30.8) | 1.56 (0.64, 3.8) |




References
- Smolinski MS, Crawley AW, Olsen JM. Finding outbreaks faster. Health Secur. 2017;15(2):215-20. Available from: http://www.liebertpub.com/doi/10.1089/hs.2016.0069. doi: 10.1089/hs.2016.0069.
- Oppenheim B, Gallivan M, Madhav NK, Brown N, Serhiyenko V, Wolfe ND, Ayscue P. Assessing global preparedness for the next pandemic: development and application of an Epidemic Preparedness Index. BMJ Glob Health. 2019;4(1):e001157. Available from: https://gh.bmj.com/lookup/doi/10.1136/bmjgh-2018-001157. doi: 10.1136/bmjgh-2018-001157.
- Wellington J, Nur A, Nicholas A, Uwishema O, Chaito H, Awosiku O, Al Tarawneh YJ, Nasser Sharafeddine JA, Onyeaka CVP, Onyeaka H. Marburg virus outbreak in Ghana: an impending crisis. Ann Med Surg (Lond). 2022;81:104377. Available from: https://journals.lww.com/10.1016/j.amsu.2022.104377. doi: 10.1016/j.amsu.2022.104377.
- Oleribe OO, Crossey MME, Taylor-Robinson SD. Nigerian response to the 2014 Ebola viral disease outbreak: lessons and cautions. Pan Afr Med J. 2015;22(Suppl 1):13. Available from: http://www.panafrican-med-journal.com/content/series/22/1/13/full. doi: 10.11604/pamj.supp.2015.22.1.6490.
- Steele L, Orefuwa E, Bino S, Singer SR, Lutwama J, Dickmann P. Earlier outbreak detection—a generic model and novel methodology to guide earlier detection supported by data from low- and mid-income countries. Front Public Health. 2020;8:452. Available from: https://www.frontiersin.org/article/10.3389/fpubh.2020.00452/full. doi: 10.3389/fpubh.2020.00452.
- Frieden TR, Lee CT, Bochner AF, Buissonnière M, McClelland A. 7-1-7: an organising principle, target, and accountability metric to make the world safer from pandemics. Lancet. 2021;398(10300):638-40. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673621012502. doi: 10.1016/S0140-6736(21)01250-2.
- Hedberg CW, Greenblatt JF, Matyas BT, Lemmings J, Sharp DJ, Skibicki RT, Liang AP. Timeliness of enteric disease surveillance in 6 US states. Emerg Infect Dis. 2008;14(2):311-3. Available from: http://wwwnc.cdc.gov/eid/article/14/2/07-0666_article.htm. doi: 10.3201/eid1402.070666.
- Altmann M, Wadl M, Altmann D, Benzler J, Eckmanns T, Krause G, Spode A, an der Heiden M. Timeliness of surveillance during outbreak of Shiga Toxin–producing Escherichia coli infection, Germany, 2011. Emerg Infect Dis. 2011;17(10):1906-9. Available from: http://wwwnc.cdc.gov/eid/article/17/10/11-1027_article.htm. doi: 10.3201/eid1710.111027.
- Nicolay N, Garvey P, Delappe N, Cormican M, McKeown P. Completeness and timeliness of Salmonella notifications in Ireland in 2008: a cross sectional study. BMC Public Health. 2010;10:568. Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-10-568. doi: 10.1186/1471-2458-10-568.
- Galanis E, Taylor M, Romanowski K, Bitzikos O, Jeyes J, Nowakowski C, Stone J, Murti M, Paccagnella A, Forsting S, Li S, Hoang L. Evaluating the timeliness of enteric disease surveillance in British Columbia, Canada, 2012-13. Can J Infect Dis Med Microbiol. 2017;2017:9854103. Available from: https://www.hindawi.com/journals/cjidmm/2017/9854103/. doi: 10.1155/2017/9854103.
- Chan EH, Brewer TF, Madoff LC, Pollack MP, Sonricker AL, Keller M, Freifeld CC, Blench M, Mawudeku A, Brownstein JS. Global capacity for emerging infectious disease detection. Proc Natl Acad Sci U S A. 2010;107(50):21701-6. Available from: https://pnas.org/doi/full/10.1073/pnas.1006219107. doi: 10.1073/pnas.1006219107.
- Impouma B, Roelens M, Williams GS, Flahault A, Codeço CT, Moussana F, Farham B, Hamblion EL, Mboussou F, Keiser O. Measuring timeliness of outbreak response in the World Health Organization African region, 2017–2019. Emerg Infect Dis. 2020;26(11):2555-64. Available from: http://wwwnc.cdc.gov/eid/article/26/11/19-1766_article.htm. doi: 10.3201/eid2611.191766.
- Ghana Health Service. Volta Region 2018 Annual Report. Accra (Ghana): Ghana Health Service; 2018.
- Ministry of Health (Ghana). Public Health Act 851. Accra (Ghana): Ministry of Health; 2012 [cited 2022 Nov 1]. 194 p. Available from: https://www.moh.gov.gh/health-sector-acts/.
- Ministry of Health (Ghana). Technical Guidelines for Integrated Disease Surveillance and Response in Ghana. 3rd ed. Accra (Ghana): Ministry of Health; 2002 [cited 2025 Oct 14]. 222 p. Available from: https://www.moh.gov.gh/wp-content/uploads/2016/02/Integrated-Disease-Surveillance-and-Response-Ghana-Guidelines.pdf.
- Nuvey FS, Edu-Quansah EP, Kuma GK, Eleeza J, Kenu E, Sackey S, Ameme D, Abakar MF, Kreppel K, Ngandolo RB, Afari E, Bonfoh B. Evaluation of the sentinel surveillance system for influenza-like illnesses in the Greater Accra region, Ghana, 2018. PLoS One. 2019;14(3):e0213627. Available from: https://dx.plos.org/10.1371/journal.pone.0213627. doi: 10.1371/journal.pone.0213627.
- Muriithi MK. The determinants of health seeking behaviour in a Nairobi slum, Kenya. Eur Sci J. 2013;9(8):151-64. Available from: https://eujournal.org/index.php/esj/article/view/884. doi: 10.19044/esj.2013.v9n8p%25p.
- Kuuire VZ, Bisung E, Rishworth A, Dixon J, Luginaah I. Health-seeking behaviour during times of illness: a study among adults in a resource poor setting in Ghana. J Public Health (Oxf). 2016;38(4):e545-53. Available from: https://academic.oup.com/jpubhealth/article-lookup/doi/10.1093/pubmed/fdv176. doi: 10.1093/pubmed/fdv176.
- Wagner MM, Gresham LS, Dato V. Case detection, outbreak detection, and outbreak characterization. In: Handbook of Biosurveillance. San Diego (CA): Elsevier; 2006. p. 27-50. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780123693785500053. doi: 10.1016/B978-012369378-5/50005-3.
- Orish VN, Maalman RSE, Donkor OY, Ceruantes BYH, Osei E, Amu H, Appiah PK, Konlan KD, Mumuni H, Kim E, Kim S, Jung H, Ofori-Amoah J, Kofie P, Adjuik M, Alhassan RK, Donkor ES, Zottor FB, Kweku M, Amuna P, Kim SY, Gyapong JO. Assessing health-seeking behaviour and malaria prevention practices among communities in four districts of the Volta Region of Ghana. Malar J. 2021;20(1):450. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-021-03986-7. doi: 10.1186/s12936-021-03986-7.
- Swaan C, van den Broek A, Kretzschmar M, Richardus JH. Timeliness of notification systems for infectious diseases: a systematic literature review. PLoS One. 2018;13(6):e0198845. Available from: https://dx.plos.org/10.1371/journal.pone.0198845. doi: 10.1371/journal.pone.0198845.
- Sodjinou VD, Ayelo PA, Salami L, Kpozehouen A, Ouendo DEM. Assessment of the earliness of detection and notification of major infectious threats to global health security in Benin. Arch Clin Biomed Res. 2022;6(1):196-208. Available from: http://www.fotunejournals.com/assessment-of-the-earliness-of-detection-and-notification-of-major-infectious-threats-to-global-health-security-in-benin.html. doi: 10.26502/acbr.50170235.
- Columbia Public Health. Time-to-event data analysis [Internet]. New York (NY): Columbia Public Health; 2025 [cited 2022 Jul 27]. Available from: https://www.publichealth.columbia.edu/research/population-health-methods/time-event-data-analysis.
