Research![]() | Volume 8, Article 35, 15 May 2025
| Volume 8, Article 35, 15 May 2025
A cross-sectional analysis of schistosomiasis case detection by rectal mucosal biopsy among patients attending the digestive endoscopy unit of the internal medicine department
Kaly Keïta1,&, Stéphane Loique Djeugoué1, Boua Daoud Camara2, Alassane Alfousséni Doumbia3, Cheick Oumar Kamissoko4, Oumar Sandji5, Adama Sinayoko1, Abdoulaye Keïta6, Maïmouna Togo7, Charles Dara3, Garan Dabo3, Abdoulaye Mamadou Traoré8,10, Ibrahima Amadou Dembélé1, Djibril Sy1,10, Djénèbou Traoré1,10, Assétou Soukho Kaya1,10, Mamadou Dembélé10, Abdoulaye Kassoum Koné9,10, Daouda Kassoum Minta8,10, Hamar Alassane Traoré10
1Department of Internal Medicine, University Hospital Center of the Point G, Bamako, Mali, 2Department of Internal Medicine, Nianankoro Fomba Hospital, Ségou, Mali, 3Department of Medicine, University Hospital Center “Hôpital du Mali”, Bamako, Mali, 4Sélingué Reference Health Center, Mali, 5Department of Medicine, Referral Health Center of Commune 1, Bamako, Mali, 6Drug and Laboratory Unit, Regional Health Department of Koulikoro, Mali, 7Department of Neurology, University Hospital Center of Gabriel Touré, Bamako, Mali, 8Department of Infectious Diseases, University Hospital Center of the Point G, Bamako, Mali, 9Malaria Research and Training Centre-International Center for Excellence in Research (MRTC-ICER), Department of Epidemiology of Parasitic Diseases, Bamako, Mali, 10Faculty of Medicine and Odontostomatology (FMOS), University of Sciences, Techniques and Technologies of Bamako (USSTB), Mali.
&Corresponding author: Dr Kaly Keïta, Department of Internal Medicine at the University Hospital Center of the Point G – Bamako, Mali: Email: keitakaly@gmail.com, hopitalpointg@hotmail.com
Received: 09 Dec 2024, Accepted: 09 May 2025, Published: 15 May 2025
Domain: Infectious Disease Epidemiology, Neglected Tropical Diseases
Keywords: Neglected tropical diseases, schistosomiasis, parasitic diseases, epidemiology, internal medicine
©Kaly Keïta et al Journal of Interventional Epidemiology and Public Health (ISSN: 2664-2824). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Kaly Keïta et al A cross-sectional analysis of schistosomiasis case detection by rectal mucosal biopsy among patients attending the digestive endoscopy unit of the internal medicine department. Journal of Interventional Epidemiology and Public Health. 2025;8:35. https://doi.org/10.37432/jieph-d-24-02023
Abstract
Introduction: The detection of schistosome eggs in stool or urine specimens is the most used technique in population-based studies, while the rectal mucosal biopsy is the commonly used technique in hospital setting. The aim of this hospital-based study was to describe the epidemiological aspects of schistosomiasis.
Methods: A cross-sectional study with retrospective data collection was conducted between January 01, 2011 to December 31, 2017 among patients who have undergone endoscopy and rectal mucosal biopsy at the digestive endoscopy Unit of internal medicine Department at the University Hospital Center Point G. We conducted univariate analysis to obtain mean and standard deviation for quantitative data and numbers and percentages for qualitative data. In the bivariate analysis, the Chi-square and Fisher´s exact tests were used to assess the statistical significance and strength of the associations between the categorical independent variables and the outcome variables. Variables with a Chi-square test with p < 0.2 at bivariate analysis were included in a multivariate logistic regression.
Results: 840 rectal mucosal biopsies reports (sex-ratio= 2.4, mean age= 30,99 ± 16, 66 years) were considered for analysis. Clinical indications were dominated by schistosomiasis related symptoms (46.0%), edematous syndrome (10.4%). 306 (36,4%) rectal mucosal biopsies were positive for Schistosoma haematobium (36.4%) and 8 (0.95%) for schistosoma mansoni. The highest positivity rates of Schistosoma haematobium and Schistosoma mansoni were observed in 2015 with 42.67% and in 2016 with 3.77%. The positivity rate of Schistosoma haematobium was 1.44 times more common in males than in females (p=0.030). The highest positivity rate of Schistosoma haematobium (90.24%) and Schistosoma mansoni (71.43%) were observed in patients under 50 years and 24 years, respectively. Factors independently associated with Schistosoma heamatobium infection positivity were: age group of 50 – 74 years (p=0.035), Malinké ethnic group (p=0.031), Soninké ethnic group (p=0.023), Segou residents (p=0.000), Mopti residents (p=0.039), pupil/student (p=0.003), trader (p=0.001) and hematuria (p=0.029).
Conclusion: The analysis of rectal mucosal biopsy showed a higher positivity rate of Schistosoma haematobium than of Schistosoma mansoni. The annual realization rate of rectal mucosal biopsy decreased from 2011 to 2017. The annual positivity rate of schistosomiasis is very disparate.
Introduction
Schistosomiasis, one of the neglected tropical diseases, is an acute and chronic parasitic disease caused by blood flukes (trematode worms) of the genus Schistosoma. The schistosome-induced morbidity is caused by eggs lodging in and damaging organs and tissues of the human host [1, 2].
Schistosomiasis is prevalent in tropical and subtropical areas, especially in poor communities without access to safe drinking water and adequate sanitation. It is estimated that at least 90% of those requiring treatment for schistosomiasis live in Africa [2]. More than 90% of these cases live in Sub-Saharan Africa [3]. Schistosomiasis is endemic in Mali [4] and the overall prevalence was estimated at 30% in 2019 [5]. The overall prevalence of Schistosoma heamatobium infection decreased from 88.0% in 2004 to 61.7% in 2010 and Schistosoma mansoni from 17.3% in 2004 to 12.7% [6]. Contrasting findings were noted in the Kalabancoro health district, with prevalence of urinary schistosomiasis at 10.83% and a prevalence of intestinal schistosomiasis at 50.83% [7].
Schistosomiasis is classically described as a rural disease, but, there has been an expansion of schistosomiasis foci towards urban areas faced with a rapid and disordered urbanization. Indeed, Dabo et al., found that there is a high transmission risk for schistosomiasis in Bamako [8].
Schistosome infection–related morbidities range from anaemia, haematuria, bloody diarrhoea and abdominal pain, to organ-specific effects such as chronic hepatosplenism, periportal fibrosis, and ureteral and/or bladder fibrosis, calcification of urinary tract and bladder cancer [9]. Other manifestations such kidney damage, genital lesions, vaginal bleeding, pain during sexual intercourse and nodules in the vulva, pathology of the seminal vesicles, prostate and infertility as long-term irreversible consequences were noted [2].
Preventive chemotherapy with praziquantel (PZQ) is the strategy recommended by the World Health Organization (WHO) for the control of schistosomiasis [10]. In Mali, the history of schistosomiasis control efforts begun with dam-building project in 1978 [11], national programme in 1982 [12], initial control program with PZQ distribution implemented by the Ministry of Health, through the National Institute of Public Health Research (INRSP), in collaboration with WHO and with funds from the German Technical Co-operation (GTZ) until 1992 [11], new initiative to resume the national control activities, technical and financial support from the Schistosomiasis Control Initiative (SCI) in 2004 [13], the schistosomiasis control programme became part of the integrated national control programme on NTDs in 2007 [14]. So, the mass drug administration (MDA) with PZQ scaling up to cover seven regions plus Bamako, targeting all school-age children and adults at high risk in hyper-endemic regions, and achieving 100% geographical coverage was ensured by Mali’s national schistosomiasis control programme (72–100% programme coverage). The preventive chemotherapy strategy is determined according to the prevalence of the infection: i) a prevalence of infection below 10% entails the administration of preventive chemotherapy every 3 years; ii) 10 to 49%, treatment every 2 years; iii) and 50% or greater, treatment annually [15].
However, few studies have monitored the progress and impact of repeated treatment on schistosomiasis in Mali. Landouré et al., found the persistent prevalence of both infections and relatively high intensity level of Schistosoma mansoni infection after repeated chemotherapy in highly-endemic Segou Region [6]. A Malian study assessed the heavy burden of schistosomiasis and associated morbidity in children after repeated chemotherapy [4].
Schistosome infection-related morbidity investigations may include haemoglobin concentrations using a portable battery-operated HemoCue® photometer [16], urine examination for micro-haematuria using urine reagent strips to detect blood in urine specimens [17], Ultrasound examination using portable ultrasonography device [18].
Schistosomiasis is diagnosed through the detection of parasite eggs in stool thick smear slides (Duplicate Kato-Katz (K-K) or urine specimens (Duplicate urine syringe filtration slides) [19, 20]. These are the mostly used techniques of detecting parasite eggs in population-based studies [6, 8, 21, 22]. Antibodies and/or antigens detected in blood or urine samples are also indications of diagnosed infection for people living in non-endemic or low-transmission areas [2]. The rectal mucosal biopsy, a valuable diagnostic tool for schistosomiasis, is essential when the parasitological examination is negative. All schistosome species emit eggs that may migrate and deposit in the rectal mucosa especially Schistosoma haematobium. Therefore, this irregular deposition is the result of peculiar venous drainage and granulomatous inflammation that traps the eggs in the rectum. The resulting chronic infection leads to granuloma formation and rectal lesions, facilitating their detection by biopsy [23]. In addition, the tissue biopsy from the bladder, and liver can be obtained to look for schistosome eggs within the tissue samples [23]. It is the most technique used in hospital setting as the three previous studies carried out in internal medicine department of the Hospital of Point G demonstrating the increasing trend of the Schistosoma haematobium prevalence but decreasing trend of the Schistosoma mansoni prevalence and others species remaining unidentifiable [24]. Furthermore, some hospital reported data found the similar observations [25-27].
Investigations of risk factors associated with schistosomiasis are crucial to identify the mechanisms for transmission and persistence of this endemic disease. Some previous studies demonstrated that certain sociodemographic and environmental factors such as river side, parents’ occupations, childhood, gender, and residing next to a water reservoir are associated with schistosomiasis [8, 7].
Since 2005, we do not know whether there is a change in the epidemiological profile of schistosomiasis and distribution of schistosome species in Mali. A research strategy that determines the positivity rate of schistosomiasis, identifies the different schistosome species in Mali, determines the frequency of clinical indications (schistosome infection-related morbidity) and explores the factors associated with schistosomiasis positivity may reveal critical information which could inform preventive and treatment strategies for the Mali’s national schistosomiasis control programme. The aim of this study hospital-based study was to describe the epidemiological aspects of schistosomiasis from January 2011 to December 2017 in the digestive endoscopy Unit of internal medicine Department at the University Hospital Center Point G, Mali. The specific objectives were to determine the socio-demographic aspects of the patients; to determine the trends of the positivity rate of schistosomiasis and the distribution of schistosome species in Mali; to determine the frequency of clinical indications (schistosome infection-related morbidity) and to determine the correlation between the independent variables and the schistosomiasis positivity.
Methods
Study design and setting
A hospital-based retrospective cross-sectional study was conducted from June 01, 2018 to April 01, 2019 to determine the trend of schistosomiasis annual positivity rate, the distribution of schistosome species, and identify the associated independent variables between 2011 and 2017. The study used data that was collected over 7 years from January 01, 2011 to December 31, 2017 in the digestive endoscopy unit of internal medicine department at the University Hospital Center of the Point G formerly known as National Hospital of the Point G, which is one of the 5 (five) public tertiary referral hospitals in Bamako, Mali’s political capital. The rectal mucosal biopsy was performed by the seven trained internists in the digestive endoscopy Unit, among patients that presented with schistosome infection-associated morbidity including those referred from different health facilities for detection of schistosome eggs via rectal mucosal biopsy.
Study population
All the patients who underwent rectal mucosal biopsy in the digestive endoscopy unit of internal medicine department at the University Hospital Center of the Point G during the study period. We excluded patients whose rectal mucosal biopsy reports were not exploitable.
Study materials and technical procedures
Rectal mucosal biopsy requires inexpensive equipment. It is easy to perform and takes less than 5 minutes, including microscopic examination. Most often, two biopsies were performed; the first, under anoscope, 7-10 cm from the anal margin (low biopsy); the second, by the rigid rectoscope, 15-20 cm from the margin (high biopsy). The two samples are crushed between two slides coated with chloral gum and then examined at low magnification. The low biopsy is perfectly adopted for the clinical screening of urinary and intestinal schistosomiasis. The diagnosis of active schistosomiasis is based on the observation of “clear” eggs., and that of non-progressive schistosomiasis is based on the observation of dark or even “black” eggs, but it is often difficult to be formal.
Variables: the independent study variables were the socio-demographic characteristics which included the patient’s sex, age, ethnic group, profession, and residence. Different schistosome species (Schistosoma mansoni, Schistosoma japonicum, Schistosoma mekongi, Schistosoma guineensis, Schistosoma intercalatum, Schistosoma haematobium) was the dependent (outcome) variable, which diagnosis was established using a rectal mucosal biopsy examination.
Definitions: The positivity rate of schistosomiasis was considered as the dependent variable, corresponding to the percentage of positive cases recorded over a period of time and is obtained by the following calculation:
Number of patients with eggs/total exams performed, multiply by 100.
The slide was considered positive when we observed “clear” eggs (active schistosomiasis) and dark or even “black” eggs (non-progressive schistosomiasis).
Data sources/ measurement
Data collection tool: A pre-established survey form was designed and used to collect data on the sociodemographic characteristics and the results of rectal mucosal biopsy from rectal mucosal biopsy registry, which also contained detailed rectal mucosal biopsy reports.
Data collection: sociodemographic variables, including sex, age, profession, residence and the rectal mucosal biopsy results were collected from rectal mucosal biopsy registry.,
Difficulties and biases: during this study, we encountered certain problems related to the lack of information in the reports of rectal mucosal biopsy. Sometimes the examinator and the prescriber did not provide all the required information in the rectal mucosal biopsy reports and the clinical information, respectively.
Sampling and sample size: this was an exhaustive sampling of all cases with rectal mucosal biopsy reports during the study period. The sample size was not calculated.
Statistical methods
The collected data were entered into SPSS version 22 software for cleaning and analysis. Data cleaning was done by checking and correcting for duplicates and completing missing data, and correcting outliers. We conducted statistical analyses using Epi Info version 7.2 and SPSS version 22 software. We used Microsoft Excel to generate bar graphs. We conducted univariate analysis to obtain mean and standard deviation for quantitative data and numbers and percentages for qualitative data. In the bivariate analysis, we calculated odds ratios (OR), 95% confidence intervals (C.I), and p-values. The outcome of interest for bivariate analysis was Schistosoma haematobium and Schistosoma mansoni infection positivity. The Chi-square and Fisher´s exact tests were used to assess the statistical significance and strength of the associations between the categorical independent variables (year of rectal mucosal biopsy realization, age, sex, ethnic group, profession, residence and clinical indication) and the outcome variables. Variables with a Chi-square test with p < 0.2 at bivariate analysis were included in a multivariate logistic regression model using backward stepwise elimination to identify independent factors associated with Schistosoma heamatobium infection positivity. Those variables with p < 0.05 were retained in the final model and considered significantly associated with Schistosoma heamatobiumschistosomiasis positivity. We did not run model for Schistosoma mansoni because of its low positivity rate.
Ethical consideration
According to the Helsinki guideline, research involving human subjects should be conducted ethically, with the well-being of the subject taking priority over scientific or societal interests. Our research study aimed to perform a cross-sectional analysis of schistosomiasis case detection by rectal mucosal biopsy among patients attending the digestive endoscopy unit of the internal medicine department involved human subjects. However, we used secondary data on patients diagnosis (main diagnostic tools as anoscope, rectoscope, biopsy forceps) and did not use the biological specimens.
In addition, the study was retrospective and all data was extracted anonymously from the rectal mucosal biopsy registry; therefore patients’ informed consent was not required. Given the nature of the study, formal ethical approval from an institutional review board/ethics committee was not sought. However, formal permission to conduct this study and access the rectal mucosal biopsy registry was obtained from the General Director of University Hospital Center of the Point G. The registry was returned to the archive room immediately after exploitation.
Results
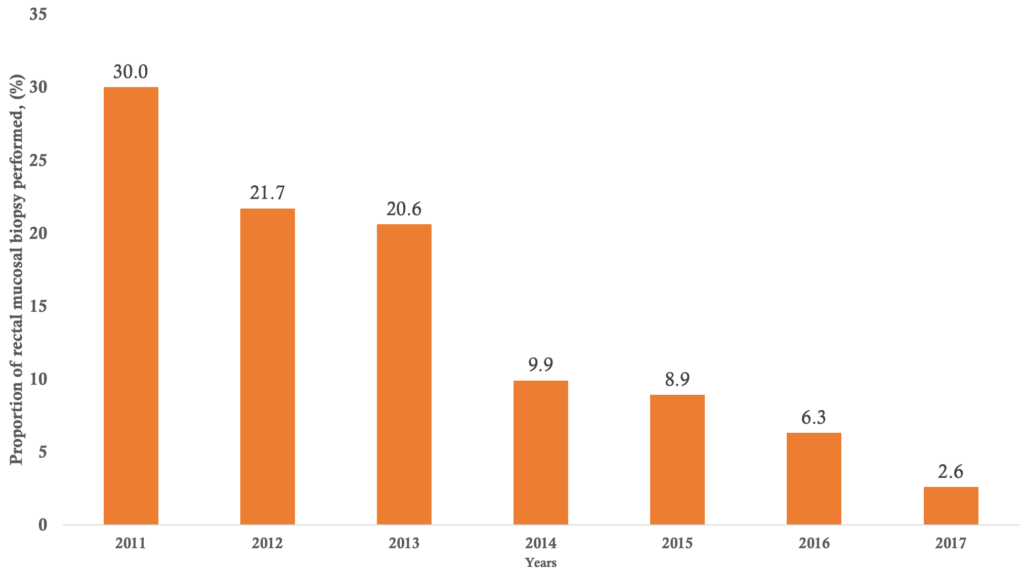
Univariate analysis of socio-demographic data: during the 7 years study period, 845 rectal mucosal biopsies were performed in the digestive endoscopy unit of internal medicine department at the University Hospital Center of the Point G. Five reports of rectal mucosal biopsy were not exploitable therefore, 840 rectal mucosal biopsies reports were considered for analysis. The distribution of patients according to the year of rectal mucosal biopsy realization is shown in figure 1. In 2011, two hundred and fifty (30% of cases) rectal mucosal biopsies were performed. The annual realization rate of rectal mucosal biopsy decreased from 2011 to 2017. On average 120 rectal mucosal biopsies were performed annually. Socio-demographic characteristics of the study patients are summarised in Table 1. Males accounted for 64.6% with a sex ratio of 2.4. Patients under 50 years represented 77.4% of the study population. The mean age of patients was 30,99 ± 16.66 years, the minimum age was 3 years and the maximum age was 85 years. The Bamanan ethnic group accounted for 18.7% of cases followed by Peulh and Soninké ethnic groups with 13.7 % of cases and 7.3% of cases respectively. The pupils/students represented 25.0% of participants, followed by farmers at 12.6% and housewives at 12.4%. Most of the patients resided in Bamako 39.4%.
Univariate analysis of clinical data: Table 2 summarizes the distribution of patients according to the clinical indications of the rectal mucosal biopsy. The schistosomiasis related symptoms (46.0% of cases) followed by edematous syndrome (10.4%) and haematuria (8.5%) were the most common indications for rectal mucosal biopsy. The rectal mucosal biopsy results are illustrated in Table 3. Out of the 840 rectal mucosal biopsies analysed for schistosoma infection, 306 rectal mucosal biopsies were positive to Schistosoma haematobium, which is a positivity rate of 36.4%. Only 8 rectal mucosal biopsies were positive to Schistosoma mansoni, a positivity rate of 0.95%.
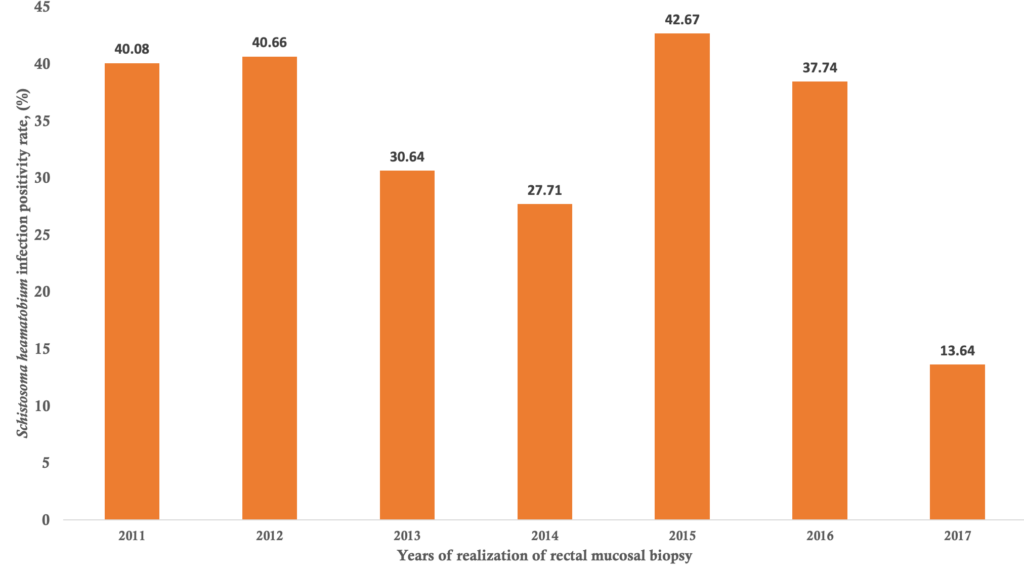
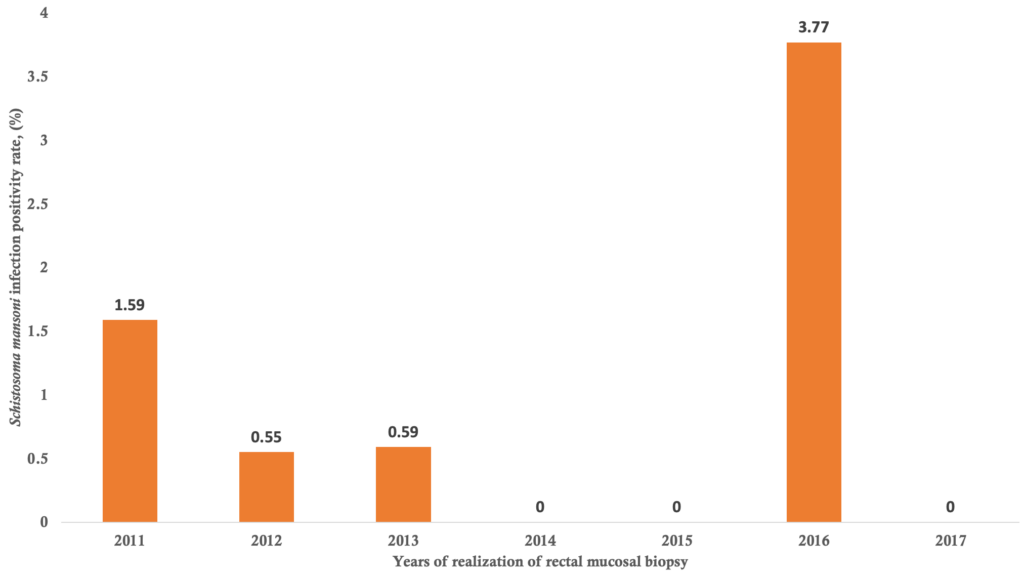
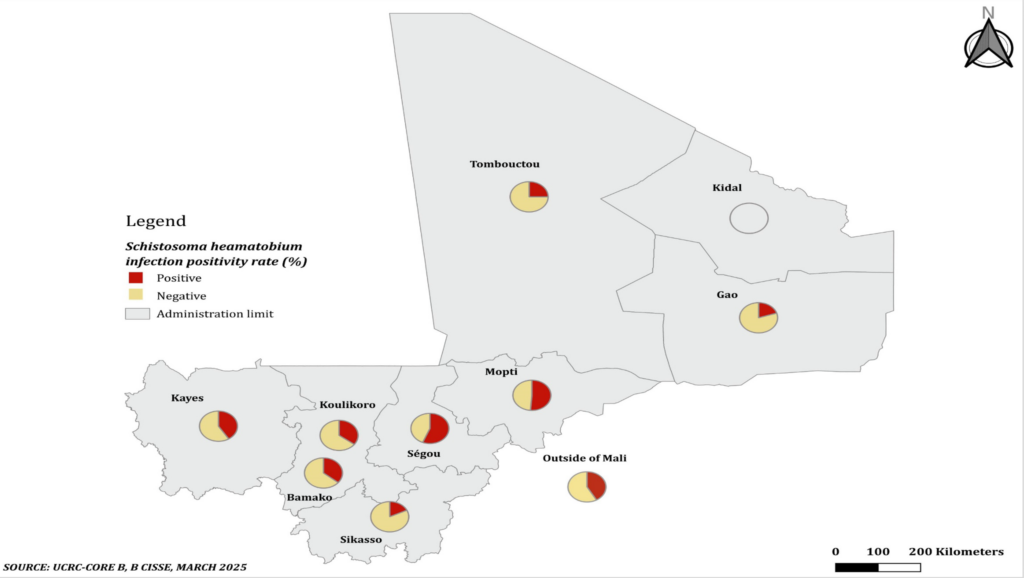
Bivariate analysis of factors associated with Schistosoma heamatobium and Schistosoma mansoni infection positivity of the rectal mucosal biopsies: Distribution of patients according to the year of rectal mucosal biopsy realization and the Schistosoma heamatobium infection positivity and Schistosoma mansoni infection positivity are summarized in Figure 2 and Figure 3, respectively. The highest positivity rates of Schistosoma haematobium and Schistosoma mansoni were observed in 2015 with 42.67% and in 2016 with 3.77% respectively, and the lowest was 13.64% for Schistosoma heamatobium in 2017 and no cases were detected in 2014, 2015 and 2017 for Schistosoma mansoni. The average annual positivity rate was 14.29 % for Schistosoma haematobium. The spatial distribution of Schistosoma heamatobium infection positivity rate by the residence is illustrated in Figure 4. The highest Schistosoma heamatobium infection positivity rate was recorded in Segou (56.25%) followed by Mopti (51.28%) and outside of the Mali (41.67%). Table 4 shows the distribution of patients according to sex and the Schistosoma heamatobiumand Schistosoma mansoni infection positivity of the rectal mucosal biopsy. The positivity rate of Schistosoma haematobium was 1.44 times more common in male than in female (214 cases versus 69 cases; OR= 1. 44; 95% CI= 1.03 – 2.01; p=0.030). There was no statistically significant difference regarding the positivity rate of Schistosoma mansoni between male and female. The distribution of patients according to the age groups and the Schistosoma heamatobium and Schistosoma mansoni infection positivity of the rectal mucosal biopsy are illustrated in Table 5. The large increase in positivity rate of Schistosoma haematobium was observed for patients under 50 years (89.24% of cases). The highest positivity rate of Schistosoma mansoni was observed for patients under than 24 years (71.43% of cases). The distribution of patients according to the clinical indications and Schistosoma heamatobium and Schistosoma mansoni infection positivity of the rectal mucosal biopsy are shown in Table 6. Schistosomiasis related symptoms were most common clinical indications for Schistosoma haematobium (51.90% cases) and splenomegaly for Schistosoma mansoni (28.57% of cases). Only haematuria was significantly associated with Schistosoma haematobium positivity (cOR=1.89, 95%CI=1.16 – 3.09, p=0.009).
Multivariate analysis of factors associated with Schistosoma heamatobium infection positivity of the rectal mucosal biopsies: Table 7 shows the results from the logistic regression analyses that included variables significantly associated with Schistosoma heamatobium infection positivity from the Chi-square test of independence. Adjusting for the factors simultaneously, two factors, Malinké ethnic group (aOR= 1.89 ; 95% CI= 1.06 – 3.38 ; p=0.031) and Soninké ethnic group (aOR= 1.89; 95% CI= 1.09 – 3.27; p=0.023) remained significantly associated with Schistosoma heamatobium infection positivity. For the profession, two factors, pupil/student (aOR= 1.69; 95% CI= 1.19 – 2.40; p=0.003) and trader (aOR= 2.42; 95% CI= 1.43 – 4.12; p=0.001) were also factors that significantly influenced Schistosoma heamatobium infection positivity. The odds of Schistosoma heamatobium infection positivity increased in Segou residents (aOR= 2.66; 95% CI= 1.63 – 4.87; p=0.000) and in Mopti residents (aOR= 2.04; 95% CI= 1.04 – 4.00; p=0.039) respectively, compared to those who did not live in Segou and Mopti. While the odds of Schistosoma heamatobium infection positivity were lower among Sikasso residents (aOR= 0.45, 95%CI= 0.22-0.87) compared to non-residents. Other significant risk factors identified were: age group of 50 – 74 years (aOR= 0.60; 95% CI= 0.37 – 0.97; p=0.035), and hematuria (aOR= 1.78; 95% CI= 1.06 – 2.99; p=0.029).
Discussion
The results from our study revealed that there were two schistosome species: Schistosoma heamatobium and Schistosoma mansoni and the former was more prevalent. We found that the annual positivity rate of schistosomiasis was disparate and its average annual positivity rate was 14.29 % for Schistosoma haematobium. The highest positivity rates of Schistosoma haematobium and Schistosoma mansoni were observed in 2015 and 2016, respectively. The Segou region recorded the highest positivity rate of Schistosoma heamatobium infection. We also found that some socio-demographic factors were independently associated with Schistosoma heamatobium schistosomiasis positivity.
This 7-year study has permitted to include 840 rectal mucosal biopsies reports, more than the three previous studies carried out in internal medicine [24]. The rectal mucosal biopsies performed were higher in 2011, this result is consistent with the study conducted by Coulibaly [27] in the same University Hospital Center of the Point G, but in the department of anatomy-pathology. The aim of this previous study was to determine the epidemiological and hisptopathological aspects of tissue schistosomiasis, indicating that the schistosomiasis was more investigated and diagnosed in both departments. In addition, the positivity rate of schistosomiasis was higher in 2015 for Schistosoma heamatobium and in 2016 forSchistosoma mansoni. In contrast, the maximum of cases was diagnosed in 2011 (40/145 cases, 27.6% of cases) according to this study [27].
This disparate distribution of annual positivity rate of Schistosoma haematobium and Schistosoma mansoni from 2011 to 2017 is in line with what was achieved in other population-based survey after repeated chemotherapy implemented in Mali since 2005 [6], in which authors noted a significant reduction in intensity of infection on both infections and modest but significant reduction in Schistosoma haematobium prevalence were achieved in highly-endemic Segou region. Both persistent positivity rate of schistosomiasis in our study and the persistent prevalence of both infections and relatively high level of intensity of Schistosoma mansoni infection in a study conducted by Landouré et al., [6]. These could be explained by the fact that the current schistosomiasis control in the integrated national non transmitted diseases (NTD) control programmes focus has been almost exclusively on preventive chemotherapy, while other components such as case management, snail control, and safe water, sanitation and hygiene are not implemented. Whereas, the comprehensive control measures are recommended by WHO include preventive chemotherapy, intensified case management, vector and intermediate host control, veterinary public health at the human-animal interface, and provision of safe water, sanitation and hygiene [28].
Most of epidemiological studies on schistosomiasis demonstrate the male predominance [26, 29-31], consistent with our study. However, some authors found a female predominance [1, 25, 27]. This discrepancy may could be explained by the fact that the study materials and the participant recruitment approaches were different. In addition, the spatial and temporal distributions of schistosomiasis are extremely variable [25, 29], depending likely on gender differences in these communities.
Several data from published works indicate that the young adults were more infected by schistosomiasis [27, 29], as were found in our study. In contrast, El-Khoby et al. showed that the schistosomiasis was more prevalent in infants aged from 0 – 10 years. This was a population-based study while ours was hospital-based, which may explain the difference.
In our study, the Bamanan ethnic group was more represented followed by Peulh and Soninké ethnic group, in consistence with previous study including data from the Demographic and Health Survey of Mali (EDSM VI) [27, 32]. Some reports revealed that housewife was more infected by schistosomiasis [24, 27], these findings parallel with our study comprising commonly pupils and students, farmers and housewifes. In addition, this finding reflects the report of Demographic and Health Survey of Mali (EDSM VI) in which the housewife is the most common occupational activities [32]. In our study, the majority of patients resided in Bamako, consistent with the study conducted in Mali by Coulibaly [27]. Both studies were conducted in Bamako, which may explain this result.
The schistosomiasis related symptoms followed by edematous syndrome and hematuria motivated rectal mucosal biopsy realization, this is not consistent with previous studies. Garango found that the edema syndrome was most common [26] and Minta et al. [24] the prolonged fever and abdominal pain. The results from our study revealed the higher positivity rate of Schistosoma heamatobium (36.4 % of cases) than of Schistosoma mansoni (0.95 % of cases). Schistosoma heamatobium and Schistosoma mansoni are the two schistosome species found in our study. These results are in line with findings from previous studies [ 6, 24, 29].
In our study, the spatial distribution of schistosomiasis positivity was markedly varied, as was found in others studies [6, 33, 34]. Few studies bivariately analyzed the sex differences in positivity rate of Schistosoma haematobium. However, several previous descriptive studies and systematic review and meta-analysis of descriptive studies reporting data distributed between male and female showed that the positivity rate of Schistosoma haematobium was more common in male than in female, this is consistent with our study findings [35-37]. Indeed, Mutapi et al. tried to answer this research question why the male is more infected than the female by comparing the humoral responses to Schistosoma haematobiumin areas with low and high levels of infection. Authors conclude that females produced significantly more anti-SEA IgG1, IgG4, IgM, anti-WWH IgE and IgG1 than male. In contrast, some authors reported a female predominance [27, 38]. The diagnostic stool, the study population, and the study setting are different among these studies, which could explain this discrepancy.
The large increase positivity rate of Schistosoma haematobium was observed for patients under than 50 years in our study notably the adolescent and adult age were a risk factor for Schistosoma haematobium, which was 1.27 times and 1.14 times more common in the < 24 years group and 25 – 49 years group, respectively, this finding parallels with the study conducted in Egypt, in which the young age group was a risk factor for Schistosoma haematobium infection it was 4.6 times and 5.5 times more in the <11 years group and 11-21 years group respectively than the older group of (36- 70 years old) [36]. Indeed, Ndhlovu et al. studying the age-related antibody profiles in Schistosoma haematobium infections in a rural community in Zimbabwe, tried to bring a rational explanation to this high prevalence of schistosomiasis in young age. Authors found that a possible association between IgE antibody responses and resistance to Schistosoma haematobium infection was indicated by a negative correlation between IgE anti-SEA levels and intensity of Schistosoma haematobium infection, and by a positive correlation between IgE responses to SEA and SWA and age [39].
In our study, the haematuria was significantly associated with Schistosoma haematobium positivity, in accordance with literature data [40].
The results from our study revealed also that the odds of Schistosoma heamatobium infection positivity increased in Segou residents and in Mopti residents respectively, compared to those who did not live in Segou and Mopti; while the odds of Schistosoma heamatobium infection positivity were lower among Sikasso residents compared to non-residents. The epidemiological setting could explain this difference as noted by Landoure et al in Mali, Schistosoma haematobium prevalence at two sites along the Niger River was almost at the baseline level; prevalence in the irrigation area showed a modest reduction for both Schistosoma haematobium and Schistosoma. mansoni; while Schistosoma haematobium prevalence in the Sahelian area showed the biggest drop [6]. This is why the integrated national non transmitted diseases (NTD) control programmes should prioritized and adapted all the public health actions against schistosomiasis according to the geographical and epidemiological data, such as the preventive chemotherapy, the case management, the snail control, and the sanitation and hygiene.
Our study has limitations. Firstly, the rectal mucosal biopsy performed outside of our study setting, which may have led to under-ascertainment on the latter. A second limitation, was the lack of some information on some of the reports of rectal mucosal biopsy since we relied on secondary data, which may have biased our findings. Finally, the study was a single-center study, which may hamper generalizability. However, this is somewhat mitigated by the fact that some of the study patients included those referred from other health facilities.
This study contains several strengths. First, it is a long-term observational study with all reports of rectal mucosal biopsy spanning a period of seven years. Second, consent procedures were not required for enrollment which eliminated non-response bias. Third, our study demonstrates the scope of the problematic of schistosomiasis in internal medicine. And finally, our data provide sufficient grounds to explore more the correlation between the infectious risk factors and schistosomiasis.
Conclusion
In internal medicine, the analysis of rectal mucosal biopsy shows a higher positivity rate of Schistosoma haematobium than of Schistosoma mansoni. Schistosoma heamatobium and Schistosoma mansoni are the two schistosome species found in our study. The annual realization rate of rectal mucosal biopsy decrease from 2011 to 2017. The annual positivity rate of schistosomiasis is very disparate. Schistosomiasis is more common in the young adults, the pupils and students, the urban residents and the males. The males are more likely than the female to be infected by Schistosoma haematobium. The adolescence and adulthood ages are significantly associated with Schistosoma haematobium positivity. A positive correlation does exist between haematuria and positivity rate of Schistosoma haematobium. A large multicentric cross sectional study is needed to more explore the correlation between the infectious risk factors and schistosomiasis.
What is already known about the topic
- Malian reports conclude that the positivity rate of Schistosoma haematobium is higher than of Schistosoma mansoni;
- Epidemiology of schistosomiasis remains less investigated.
What this study adds
- The positivity rate of Schistosoma haematobium remains higher than of Schistosoma mansoni;
- Schistosomiasis is more common in young adults, pupils and students, urban residents and males.
- The males are more likely than the females to be infected by Schistosoma haematobium; the adolescent and adult ages are significantly associated with Schistosoma haematobium positivity; and the positive correlation does exist between haematuria and positivity rate of Schistosoma haematobium.
Authors´ contributions
Conception: KK, DKM, HAT. Design of the study: KK, SLD, BDC, AAD, AKK. Data collection: COK, OS, AS, AK, MT, CD, KK. Data analysis and interpretation: IAD, DS, DT, AK, KK, AMT. Supervision: ASK. Writing original draft: KK, GD, AMT, IAD, DS, DT. Writing review and editing: KK, SLD, BDC, AAD, GD, AMT, IAD, DS, DT, ASK, MD, AKK, DKM, HAT. Guarantor of the study: HAT.
All the authors have read and agreed to the final manuscript.
| Socio-demographic characteristics | Number | Percentage (%) |
|---|---|---|
| Sex | ||
| Male | 543 | 64.6 |
| Female | 222 | 26.4 |
| No information | 75 | 8.9 |
| Age group | ||
| ≤24 years | 323 | 38.5 |
| 25 – 49 years | 327 | 38.9 |
| 50 – 74 years | 119 | 14.2 |
| ≥ 75 years | 9 | 1.1 |
| No information | 62 | 7.4 |
| Ethnic group | ||
| Bamanan | 157 | 18.7 |
| Peulh | 115 | 13.7 |
| Soninké | 61 | 7.3 |
| Malinké | 54 | 6.4 |
| Sonrai | 23 | 2.7 |
| Dogon | 21 | 2.5 |
| Senofo | 11 | 1.3 |
| No information | 364 | 43.3 |
| Others | 34 | 4.0 |
| Profession | ||
| Pupil/Student | 210 | 25.0 |
| Farmer | 106 | 12.6 |
| Housewife | 104 | 12.4 |
| Trader | 68 | 8.1 |
| Civil servant | 57 | 6.8 |
| Military | 16 | 1.9 |
| Not employed | 5 | 0.6 |
| No information | 152 | 18.1 |
| Others | 122 | 14.5 |
| Residence | ||
| Bamako | 331 | 39.4 |
| Kayes | 105 | 12.5 |
| Koulikoro | 105 | 12.5 |
| Ségou | 80 | 9.5 |
| Sikasso | 67 | 8.0 |
| Mopti | 39 | 4.6 |
| Tombouctou | 16 | 1.9 |
| Gao | 15 | 1.8 |
| Outside of Mali | 36 | 4.3 |
| No information | 46 | 5.5 |
| Clinical indications | Number | Percentage |
|---|---|---|
| Schistosomiasis related symptomsa | 386 | 46.0 |
| Edematous syndrome | 87 | 10.4 |
| Haematuria | 71 | 8.5 |
| Splenomegaly | 51 | 6.1 |
| Hepatosplenomegaly | 18 | 2.1 |
| Rectal bleeding | 16 | 1.9 |
| Anemic syndrome | 8 | 1.0 |
| Hepatomegaly | 3 | 0.4 |
| Portal hypertension syndrome | 3 | 0.4 |
| Others | 148 | 17.6 |
| No information | 49 | 5.8 |
| Total | 840 | 100.0 |
| Rectal mucosal biopsy results | Schistosoma haematobium | Schistosoma mansoni | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Positive | 306 | 36.4 | 8 | 0.95 |
| Negative | 534 | 63.6 | 832 | 99.05 |
| Total | 840 | 100.0 | 840 | 100.0 |
| Schistosoma positivity | Sex | OR (95% CI) | p-value | |
|---|---|---|---|---|
| Male, n (%) | Female, n (%) | |||
| Schistosoma haematobium – Positive | 214 (39.41) | 69 (31.08) | 1.44 (1.03 – 2.01) | 0.030 |
| Schistosoma haematobium – Negative | 329 (60.59) | 153 (68.92) | ||
| Schistosoma mansoni – Positive | 6 (1.10) | 2 (0.90) | 1.23 (0.25 – 6.14) | 1.000 |
| Schistosoma mansoni – Negative | 537 (98.9) | 220 (99.10) | ||
| Age group | Infection status | Schistosoma haematobium | Schistosoma mansoni | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive, n (%) | Negative, n (%) | OR (95% CI) | p-value | Positive, n (%) | Negative, n (%) | OR (95% CI) | p-value | ||
| < 24 years | Yes | 130 (45.14) | 158 (54.86) | 1.27 (0.94 – 1.70) | 0.116 | 5 (71.43) | 2 (28.57) | 3.56 (0.69 – 18.47) | 0.134 |
| No | 193 (39.39) | 297 (60.61) | 1 | 318 (41.25) | 453 (58.75) | 1 | |||
| 25 – 49 years | Yes | 127 (44.10) | 161 (55.90) | 1.14 (0.85 – 1.54) | 0.371 | 0 (0.00) | 7 (100.00) | 0.023 | |
| No | 200 (40.82) | 290 (59.18) | 1 | 327 (42.41) | 444 (57.59) | ||||
| 50 – 74 years | Yes | 29 (10.07) | 259 (89.93) | 0.49 (0.32 – 0.78) | 0.002 | 2 (28.57) | 5 (71.43) | 2.24 (0.43 – 11.66) | 0.291 |
| No | 90 (18.37) | 400 (81.63) | 1 | 117 (15.18) | 654 (84.82) | 1 | |||
| > 75 years | Yes | 2 (0.69) | 286 (99.31) | 0.48 (0.09 – 2.34) | 0.497 | 0 (0.00) | 7 (100.00) | 1.000 | |
| No | 7 (1.43) | 483 (98.57) | 1 | 9 (1.17) | 762 (98.83) | 1 | |||
| Clinical Indications | Schistosoma Haematobium | Schistosoma Mansoni | ||||||
|---|---|---|---|---|---|---|---|---|
| Positive, n (%) | Negative, n (%) | Crude OR (95% CI) | p-value | Positive, n (%) | Negative, n (%) | Crude OR (95% CI) | p-value | |
| Hematuria (yes) | 36 (12.46) | 35 (6.97) | 1.89 (1.16 – 3.09) | 0.009 | 1 (14.29) | 70 (8.93) | 1.70 (0.20 – 14.32) | 0.484 |
| 253 (87.54) | 467 (93.03) | 6 (85.71) | 714 (91.07) | 1 | ||||
| Rectal bleeding (yes) | 3 (1.04) | 13 (2.59) | 0.39 (0.11 – 1.39) | 0.136 | 0 (0.00) | 16 (2.04) | 1.000 | |
| 286 (98.96) | 489 (97.41) | 7 (100.00) | 768 (97.94) | |||||
| Edematous syndrome (yes) | 31 (10.73) | 56 (11.16) | 0.96 (0.60 – 1.52) | 0.852 | 1 (14.29) | 86 (10.97) | 1.35 (0.16 – 11.37) | 0.559 |
| 258 (89.27) | 446 (88.84) | 6 (85.71) | 698 (89.03) | |||||
| Hepatomegaly (yes) | 1 (0.35) | 2 (0.40) | 0.87 (0.01 – 9.62) | 1.000 | 0 (0.00) | 3 (0.38) | 1.000 | |
| 288 (99.65) | 500 (99.60) | 7 (100.00) | 781 (99.62) | |||||
| Splenomegaly (yes) | 14 (4.84) | 37 (7.37) | 0.64 (0.34 – 1.20) | 0.164 | 2 (28.57) | 49 (6.25) | 6.00 (1.14 – 31.72) | 0.069 |
| 275 (95.16) | 465 (92.63) | 5 (71.43) | 735 (93.75) | |||||
| Hepatosplenomegaly (yes) | 4 (1.38) | 14 (2.79) | 0.49 (0.16 – 1.50) | 0.202 | 1 (14.29) | 17 (2.17) | 7.52 (0.86 – 65.91) | 0.149 |
| 285 (98.62) | 488 (97.21) | 6 (85.71) | 767 (97.83) | |||||
| Schistosomiasis related symptoms (yes) | 150 (51.90) | 236 (47.01) | 1.22 (0.91 – 1.63) | 0.185 | 0 (0.00) | 386 (49.23) | 0.015 | |
| 139 (48.10) | 266 (52.99) | 7 (100.00) | 398 (50.77) | |||||
| Portal hypertension syndrome (yes) | 0 (0.00) | 3 (0.60) | – | 0.558 | 0 (0.00) | 3 (0.38) | 1.000 | |
| 289 (100.00) | 499 (99.40) | 7 (100.00) | 781 (99.62) | |||||
| Anemic syndrome (yes) | 1 (0.35) | 7 (1.39) | 0.25 (0.03 – 2.00) | 0.269 | 0 (0.00) | 8 (1.02) | 1.000 | |
| 288 (99.65) | 495 (98.61) | 7 (100.00) | 776 (98.98) | |||||
| Others (yes) | 49 (16.96) | 99 (19.72) | 0.83 (0.57 – 1.21) | 0.337 | 2 (28.57) | 146 (18.62) | 1.75 (0.33 – 9.09) | 0.621 |
| 240 (83.04) | 403 (80.28) | 5 (71.43) | 638 (81.38) | |||||
| Variablesa | Positive, n (%) | Negative, n (%) | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Ethnic Group | ||||||
| Malinké | 26 (8.50) | 28 (5.24) | 1.68 (0.96 – 2.91) | 0.064 | 1.89 (1.06 – 3.38) | 0.031 |
| Soninké | 32 (10.46) | 29 (5.43) | 2.03 (1.20 – 3.43) | 0.006 | 1.89 (1.09 – 3.27) | 0.023 |
| Profession | ||||||
| Pupil/Student | 93 (30.39) | 117 (21.91) | 1.56 (1.13 – 2.14) | 0.006 | 1.69 (1.19 – 2.40) | 0.003 |
| Trader | 38 (12.42) | 30 (5.62) | 2.38 (1.44 – 3.93) | 0.001 | 2.42 (1.43 – 4.12) | 0.001 |
| Civil servant | 13 (4.25) | 44 (8.24) | 0.49 (0.26 – 0.93) | 0.027 | ||
| Residence | ||||||
| Sikasso | 12 (3.92) | 55 (10.30) | 0.36 (0.18 – 0.67) | 0.001 | 0.45 (0.22 – 0.87) | 0.017 |
| Ségou | 45 (14.71) | 35 (6.55) | 2.46 (1.54 – 3.92) | 0.000 | 2.66 (1.63 – 4.87) | 0.000 |
| Mopti | 20 (6.54) | 19 (3.56) | 1.89 (0.99 – 3.61) | 0.048 | 2.04 (1.04 – 4.00) | 0.039 |
| Gao | 3 (0.98) | 12 (3.56) | 0.43 (0.12 – 1.54) | 0.182 | ||
| Sex | ||||||
| Male | 214 (39.41) | 69 (31.08) | 1.44 (1.03 – 2.01) | 0.030 | ||
| Age Group | ||||||
| < 24 years | 130 (45.14) | 193 (39.39) | 1.26 (0.94 – 1.69) | 0.116 | ||
| 50 – 74 years | 29 (10.07) | 90 (18.37) | 0.49 (0.31 – 0.78) | 0.002 | 0.60 (0.37 – 0.97) | 0.035 |
| Clinical Indications | ||||||
| Hematuria | 36 (12.46) | 35 (6.97) | 1.90 (1.16 – 3.09) | 0.009 | 1.78 (1.06 – 2.99) | 0.029 |
| Rectal bleeding | 3 (1.04) | 13 (2.59) | 0.39 (0.11 – 1.40) | 0.136 | ||
| Splenomegaly | 14 (4.84) | 37 (7.37) | 0.64 (0.34 – 1.21) | 0.164 | ||
| Schistosomiasis-related symptomsb | 150 (51.90) | 236 (47.01) | 1.21 (0.92 – 1.61) | 0.185 | ||
| Year of Biopsy | ||||||
| 2011 | 101 (33.01) | 151 (28.28) | 1.25 (0.92 – 1.69) | 0.150 | ||
| 2012 | 74 (24.18) | 108 (20.22) | 1.26 (0.89 – 1.76) | 0.180 | ||
| 2013 | 53 (17.32) | 120 (22.47) | 0.72 (0.50 – 1.04) | 0.076 | 0.72 (0.49 – 1.05) | 0.089 |
| 2014 | 23 (7.52) | 60 (11.24) | 0.64 (0.39 – 1.06) | 0.082 | 0.64 (0.38 – 1.08) | 0.096 |
| 2017 | 3 (0.98) | 19 (3.56) | 0.26 (0.07 – 0.91) | 0.024 | 0.26 (0.07 – 0.90) | 0.035 |
a Variables with p-value < 0.20 included in model.
b Schistosomiasis-related symptoms: infectious work-up, and suspicion of schistosomiasis.
References
- Gu K, Li Y, Driguez P, Zeng Q, Yu X, Sun H, Cai L, He Y, Wang W, McManus DP. Clinical diagnostic value of viable Schistosoma japonicum eggs detected in host tissues. BMC Infect Dis. 2017;17(1):244. Available from: http://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-017-2362-4
- World Health Organization. Schistosomiasis. Geneva (Switzerland): World Health Organization; 2023 [cited 2025 May 12]. Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis
- Donohue R, Mashoto K, Mubyazi G, Madon S, Malecela M, Michael E. Biosocial determinants of persistent schistosomiasis among schoolchildren in Tanzania despite repeated treatment. Trop Med Infect Dis. 2017;2(4):61. Available from: https://www.mdpi.com/2414-6366/2/4/61
- Mutombo N, Landouré A, Man WY, Fenwick A, Dembélé R, Sacko M, Keita AD, Traoré MS, Webster JP, McLaws ML. The association between child Schistosoma spp. infections and morbidity in an irrigated rice region in Mali: a localized study. Acta Trop. 2019;199:105115. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0001706X17314286
- Bintou LY, Yaro AS, Sodio B, Sacko M. Persistance de la schistosomiase urinaire en zones endémiques soumises aux traitements de masse répétés au Mali. Int J Biol Chem Sci. 2019;13(1):369-76. Available from: https://www.ajol.info/index.php/ijbcs/article/view/186741
- Landouré A, Dembélé R, Goita S, Kané M, Tuinsma M, Sacko M, Toubali E, French MD, Keita AD, Fenwick A, Traoré MS, Zhang Y. Significantly reduced intensity of infection but persistent prevalence of schistosomiasis in a highly endemic region in Mali after repeated treatment. PLoS Negl Trop Dis. 2012;6(7):e1774. Available from: https://dx.plos.org/10.1371/journal.pntd.0001774
- Maïga FK, Sangare M, Dolo H, Dicko I, Diabate AF, Keita M, Diarra L, Soumaoro L, Thera S, Diallo O, Guindo I, Traoré M, Faye O, Doumbia S, Coulibaly YI. Knowledge and factors influencing schistosomiasis control interventions in the hyperendemic health district of Kalabancoro in Mali, 2020. Pan Afr Med J. 2022;43:48. Available from: https://www.panafrican-med-journal.com/content/article/43/48/full
- Dabo A, Diarra AZ, Machault V, Touré O, Niambélé DS, Kanté A, Ongoiba A, Doumbo O. Urban schistosomiasis and associated determinant factors among school children in Bamako, Mali, West Africa. Infect Dis Poverty. 2015;4:4. Available from: https://idpjournal.biomedcentral.com/articles/10.1186/2049-9957-4-4
- Buonfrate D, Ferrari TCA, Adegnika AA, Stothard JR, Gobbi FG. Human schistosomiasis. Lancet. 2025;405(10479):658-70. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673624028149
- Montresor A, Engels D, Ramsan M, Foum A, Savioli L. Field test of the ‘dose pole’ for praziquantel in Zanzibar. Trans R Soc Trop Med Hyg. 2002;96(3):323-4. Available from: https://academic.oup.com/trstmh/article-lookup/doi/10.1016/S0035-9203(02)90111-2
- Brinkmann UK, Werler C, Traoré M, Korte R. The national schistosomiasis control programme in Mali, objectives, organization, results. Trop Med Parasitol. 1988;39(2):157-61. PMID: 3140357
- Clements ACA, Bosqué-Oliva E, Sacko M, Landouré A, Dembélé R, Traoré M, Coulibaly G, Gabrielli AF, Fenwick A, Brooker S. A comparative study of the spatial distribution of schistosomiasis in Mali in 1984-1989 and 2004-2006. PLoS Negl Trop Dis. 2009;3(5):e431. Available from: https://dx.plos.org/10.1371/journal.pntd.0000431
- Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, Zhang Y, Garba A, Stothard JR, Gabrielli AF, Clements ACA, Kabatereine NB, Toure S, Dembele R, Nyandindi U, Mwansa J, Koukounari A. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology. 2009;136(13):1719-30. Available from: https://www.cambridge.org/core/product/identifier/S0031182009990400/type/journal_article
- Dembélé M, Bamani S, Dembélé R, Traoré MO, Goita S, Traoré MN, Sidibe AK, Sam L, Tuinsma M, Toubali E, MacArthur C, Baker SK, Zhang Y. Implementing preventive chemotherapy through an integrated national neglected tropical disease control program in Mali. PLoS Negl Trop Dis. 2012;6(3):e1574. Available from: https://dx.plos.org/10.1371/journal.pntd.0001574
- World Health Organization. Helminth control in school age children: a guide for managers of control programmes. 2nd ed. Geneva (Switzerland): World Health Organization; 2011 [cited 2025 May 12]. 75 p. Available from: https://iris.who.int/bitstream/handle/10665/44671/9789241548267_eng.pdf?sequence=1
- von Schenck H, Falkensson M, Lundberg B. Evaluation of “HemoCue,” a new device for determining hemoglobin. Clin Chem. 1986;32(3):526-9. Available from: https://academic.oup.com/clinchem/article/32/3/526/5652372
- French MD, Rollinson D, Basáñez MG, Mgeni AF, Khamis IS, Stothard JR. School-based control of urinary schistosomiasis on Zanzibar, Tanzania: monitoring micro-haematuria with reagent strips as a rapid urological assessment. J Pediatr Urol. 2007;3(5):364-8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1477513107002306
- Gouvras AN, Kariuki C, Koukounari A, Norton AJ, Lange CN, Ireri E, Fenwick A, Mkoji GM, Webster JP. The impact of single versus mixed Schistosoma haematobium and S. mansoni infections on morbidity profiles amongst school-children in Taveta, Kenya. Acta Trop. 2013;128(2):309-17. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0001706X13000028
- Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14(6):397-400. PMID: 4675644
- Peters PA, Warren KS, Mahmoud AA. Rapid, accurate quantification of schistosome eggs via nuclepore filters. J Parasitol. 1976;62(1):154-5. Available from: https://www.jstor.org/stable/3279081?origin=crossref
- Ekpo UF, Oluwole AS, Abe EM, Etta HE, Olamiju F, Mafiana CF. Schistosomiasis in infants and pre-school-aged children in sub-Saharan Africa: implication for control. Parasitology. 2012;139(7):835-41. Available from: https://www.cambridge.org/core/product/identifier/S0031182012000029/type/journal_article
- Dabo A, Badawi HM, Bary B, Doumbo OK. Urinary schistosomiasis among preschool-aged children in Sahelian rural communities in Mali. Parasites Vectors. 2011;4:21. Available from: https://parasitesandvectors.biomedcentral.com/articles/10.1186/1756-3305-4-21
- Ottolino C, Atencio MH. Nuevos caminos para el diagnostico clinico preciso de la Schistosomiasis mansoni [New approaches to accurate clinical diagnosis of Schistosomiasis mansoni]. Eurekamag. [cited 2025 May 12]. Available from: https://eurekamag.com/research/023/204/023204934.php#references
- Minta DK, Dembele M, Diarra AS, et al. La morbidité bilharzienne en milieu hospitalier bamakois de l’hôpital du point G – Mali [Schistosomiasis morbidity in the hospital ward of Hospital of Point G in Bamako – Mali]. Mali Med. 2005;20(4):34-9. Available from: https://malimedical.org/2005/p34d.pdf
- Diarra FS. Distribution spatiale de la schistosomiase en milieux scolaires urbain et périurbain du district de Bamako: cas des communes situées sur la rive gauche du fleuve Niger [Spatial distribution of schistosomiasis in the municipalities of Bamako located on the left bank of the Niger river] [dissertation]. Bamako (Mali): Université des Sciences, des Techniques et des Technologies de Bamako; 2012 [cited 2025 May 13]. 61 p. Available from: https://www.bibliosante.ml/bitstream/handle/123456789/1777/13M43.pdf?sequence=1&isAllowed=y
- Garango A. La bilharziose au cours du syndrome néphrotique dans le service de Néphrologie et d’Hémodialyse du CHU Point G [Bilharzia during nephrotic syndrome in the Nephrology and Hemodialysis Department of CHU Point G] [dissertation]. Bamako (Mali): Université de Bamako; 2008 [cited 2025 May 13]. 90 p. Available from: https://www.bibliosante.ml/bitstream/handle/123456789/8681/08M60.pdf?sequence=1&isAllowed=y
- Coulibaly BM. La schistosomiase tissulaire au Mali: à propos de 145 cas au service d’anatomie et cytologie pathologiques [Tissue schistosomiasis in Mali: 145 cases in the pathological anatomy and cytology department] [dissertation]. Bamako (Mali): Université des Sciences, des Techniques et des Technologies de Bamako; 2012 [cited 2025 May 13]. 72 p. Available from: https://www.bibliosante.ml/bitstream/handle/123456789/1544/13M101.pdf?sequence=1
- World Health Organization. Accelerating work to overcome the global impact of neglected tropical diseases – a roadmap for implementation. Geneva (Switzerland): World Health Organization; 2012 [cited 2025 May 12]. 22 p. Available from: https://www.who.int/publications/i/item/WHO-HTM-NTD-2012.1
- El-Khoby T, Galal N, Fenwick A, Barakat R, El-Hawey A, Nooman Z, Habib M, Abdel-Wahab F, Gabr NS, Hammam HM, Hussein MH, Mikhail NN, Cline BL, Strickland GT. The epidemiology of schistosomiasis in Egypt: summary findings in nine governorates. Am J Trop Med Hyg. 2000;62(2 Suppl):88-99. Available from: https://www.ajtmh.org/view/journals/tpmd/62/2_suppl/article-p88.xml
- Aryeetey ME, Wagatsuma Y, Yeboah G, Asante M, Mensah G, Nkrumah FK, Kojima S. Urinary schistosomiasis in southern Ghana: 1. Prevalence and morbidity assessment in three (defined) rural areas drained by the Densu river. Parasitol Int. 2000;49(2):155-63. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1383576900000441
- Der EM, Quayson SE, Mensah JE, Tettey Y. Tissue schistosomiasis in Accra Ghana: a retrospective histopathologic review at the Korle-Bu Teaching Hospital (2004-2011). Pathol Discov. 2015;3:1. Available from: http://www.hoajonline.com/pathology/2052-7896/3/1
- Institut National de la Statistique (Mali). Enquête Démographique et de Santé (EDSM-VI) Mali 2018 [Demographic and Health Survey (EDSM-VI) Mali 2018]. Bamako (Mali): Institut National de la Statistique; 2022 [cited 2025 May 12]. Available from: https://microdata-catalog.afdb.org/index.php/catalog/140/variable/F7/V15883?name=MV844
- Amarir F, El Mansouri B, Fellah H, Sebti F, Mohammed L, Handali S, Wilkins P, El Idrissi AL, Sadak A, Rhajaoui M. National serologic survey of haematobium schistosomiasis in Morocco: evidence for elimination. Am J Trop Med Hyg. 2011;84(1):15-9. Available from: https://www.ajtmh.org/view/journals/tpmd/84/1/article-p15.xml
- Mendes RJDA, Cantanhede SPD, Pereira Filho AA, Nogueira ADJL, Silva IPD, Rosa IG. Spatial distribution of the positivity of Schistosomiasis mansoni in Maranhão State, Northeastern Brazil, from 2007 to 2016. Rev Inst Med Trop Sao Paulo. 2022;64:e53. Available from: https://www.scielo.br/j/rimtsp/a/CmTm8QDkSnp89MmbL7zts5g/?lang=en#
- Tay SCK, Amankwa R, Gbedema SY. Prevalence of Schistosoma haematobium infection in Ghana: a retrospective case study in Kumasi. Int J Parasitol Res. 2011;3(2):48-52. Available from: http://www.bioinfopublication.org/viewhtml.php?artid=BIA0001055
- Yameny A. Schistosomiasis haematobium prevalence and risk factors in El-Fayoum governorate, Egypt. J Biosci Appl Res. 2017;3(4):191-201. Available from: https://jbaar.journals.ekb.eg/article_126150.html
- Ayabina DV, Clark J, Bayley H, Lamberton PHL, Toor J, Hollingsworth TD. Gender-related differences in prevalence, intensity and associated risk factors of Schistosoma infections in Africa: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15(11):e0009083. Available from: https://dx.plos.org/10.1371/journal.pntd.0009083
- Gbalégba NGC, Silué KD, Ba O, Ba H, Tian-Bi NTY, Yapi GY, Kaba A, Koné B, Utzinger J, Koudou BG. Prevalence and seasonal transmission of Schistosoma haematobium infection among school-aged children in Kaedi town, southern Mauritania. Parasites Vectors. 2017;10:353. Available from: http://parasitesandvectors.biomedcentral.com/articles/10.1186/s13071-017-2284-4
- Ndhlovu P, Cadman H, Vennervald BJ, Christensen NØ, Chidimu M, Chandiwana SK. Age-related antibody profiles in Schistosoma haematobium infections in a rural community in Zimbabwe. Parasite Immunol. 1996;18(4):181-91. Available from: https://onlinelibrary.wiley.com/doi/10.1046/j.1365-3024.1996.d01-78.x
- Carbonell C, Rodríguez-Alonso B, López-Bernús A, Almeida H, Galindo-Pérez I, Velasco-Tirado V, Marcos M, Pardo-Lledías J, Belhassen-García M. Clinical spectrum of schistosomiasis: an update. J Clin Med. 2021;10(23):5521. Available from: https://www.mdpi.com/2077-0383/10/23/5521
Menu, Tables and Figures
On Pubmed
- Kaly Keïta
- Stéphane Loique Djeugoué
- Boua Daoud Camara
- Alassane Alfousséni Doumbia
- Cheick Oumar Kamissoko
- Oumar Sandji
- Adama Sinayoko
- Abdoulaye Keïta
- Maïmouna Togo
- Charles Dara
- Garan Dabo
- Abdoulaye Mamadou Traoré
- Ibrahima Amadou Dembélé
- Djibril Sy
- Djénèbou Traoré
- Assétou Soukho Kaya
- Mamadou Dembélé
- Abdoulaye Kassoum Koné
- Daouda Kassoum Minta
- Hamar Alassane Traoré
On Google Scholar
- Kaly Keïta
- Stéphane Loique Djeugoué
- Boua Daoud Camara
- Alassane Alfousséni Doumbia
- Cheick Oumar Kamissoko
- Oumar Sandji
- Adama Sinayoko
- Abdoulaye Keïta
- Maïmouna Togo
- Charles Dara
- Garan Dabo
- Abdoulaye Mamadou Traoré
- Ibrahima Amadou Dembélé
- Djibril Sy
- Djénèbou Traoré
- Assétou Soukho Kaya
- Mamadou Dembélé
- Abdoulaye Kassoum Koné
- Daouda Kassoum Minta
- Hamar Alassane Traoré
Navigate this article
Tables
| Socio-demographic characteristics | Number | Percentage (%) |
|---|---|---|
| Sex | ||
| Male | 543 | 64.6 |
| Female | 222 | 26.4 |
| No information | 75 | 8.9 |
| Age group | ||
| ≤24 years | 323 | 38.5 |
| 25 – 49 years | 327 | 38.9 |
| 50 – 74 years | 119 | 14.2 |
| ≥ 75 years | 9 | 1.1 |
| No information | 62 | 7.4 |
| Ethnic group | ||
| Bamanan | 157 | 18.7 |
| Peulh | 115 | 13.7 |
| Soninké | 61 | 7.3 |
| Malinké | 54 | 6.4 |
| Sonrai | 23 | 2.7 |
| Dogon | 21 | 2.5 |
| Senofo | 11 | 1.3 |
| No information | 364 | 43.3 |
| Others | 34 | 4.0 |
| Profession | ||
| Pupil/Student | 210 | 25.0 |
| Farmer | 106 | 12.6 |
| Housewife | 104 | 12.4 |
| Trader | 68 | 8.1 |
| Civil servant | 57 | 6.8 |
| Military | 16 | 1.9 |
| Not employed | 5 | 0.6 |
| No information | 152 | 18.1 |
| Others | 122 | 14.5 |
| Residence | ||
| Bamako | 331 | 39.4 |
| Kayes | 105 | 12.5 |
| Koulikoro | 105 | 12.5 |
| Ségou | 80 | 9.5 |
| Sikasso | 67 | 8.0 |
| Mopti | 39 | 4.6 |
| Tombouctou | 16 | 1.9 |
| Gao | 15 | 1.8 |
| Outside of Mali | 36 | 4.3 |
| No information | 46 | 5.5 |
Table 1: Distribution of patients according to the socio-demographic data (N= 840)
| Clinical indications | Number | Percentage |
|---|---|---|
| Schistosomiasis related symptomsa | 386 | 46.0 |
| Edematous syndrome | 87 | 10.4 |
| Haematuria | 71 | 8.5 |
| Splenomegaly | 51 | 6.1 |
| Hepatosplenomegaly | 18 | 2.1 |
| Rectal bleeding | 16 | 1.9 |
| Anemic syndrome | 8 | 1.0 |
| Hepatomegaly | 3 | 0.4 |
| Portal hypertension syndrome | 3 | 0.4 |
| Others | 148 | 17.6 |
| No information | 49 | 5.8 |
| Total | 840 | 100.0 |
aSchistosomiasis related symptoms comprised non-specified clinical indications such as infectious work-up and suspicion of schistosomiasis.
Table 2: Distribution of patients according to the clinical indications (N=840)
| Rectal mucosal biopsy results | Schistosoma haematobium | Schistosoma mansoni | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Positive | 306 | 36.4 | 8 | 0.95 |
| Negative | 534 | 63.6 | 832 | 99.05 |
| Total | 840 | 100.0 | 840 | 100.0 |
Table 3: Distribution of patients according to the rectal mucosal biopsy results
| Schistosoma positivity | Sex | OR (95% CI) | p-value | |
|---|---|---|---|---|
| Male, n (%) | Female, n (%) | |||
| Schistosoma haematobium – Positive | 214 (39.41) | 69 (31.08) | 1.44 (1.03 – 2.01) | 0.030 |
| Schistosoma haematobium – Negative | 329 (60.59) | 153 (68.92) | ||
| Schistosoma mansoni – Positive | 6 (1.10) | 2 (0.90) | 1.23 (0.25 – 6.14) | 1.000 |
| Schistosoma mansoni – Negative | 537 (98.9) | 220 (99.10) | ||
Table 4: Distribution of patients according to the sex and the Schistosoma haematobium and Schistosoma mansoni infection positivity of the rectal mucosal biopsy
| Age group | Infection status | Schistosoma haematobium | Schistosoma mansoni | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive, n (%) | Negative, n (%) | OR (95% CI) | p-value | Positive, n (%) | Negative, n (%) | OR (95% CI) | p-value | ||
| < 24 years | Yes | 130 (45.14) | 158 (54.86) | 1.27 (0.94 – 1.70) | 0.116 | 5 (71.43) | 2 (28.57) | 3.56 (0.69 – 18.47) | 0.134 |
| No | 193 (39.39) | 297 (60.61) | 1 | 318 (41.25) | 453 (58.75) | 1 | |||
| 25 – 49 years | Yes | 127 (44.10) | 161 (55.90) | 1.14 (0.85 – 1.54) | 0.371 | 0 (0.00) | 7 (100.00) | 0.023 | |
| No | 200 (40.82) | 290 (59.18) | 1 | 327 (42.41) | 444 (57.59) | ||||
| 50 – 74 years | Yes | 29 (10.07) | 259 (89.93) | 0.49 (0.32 – 0.78) | 0.002 | 2 (28.57) | 5 (71.43) | 2.24 (0.43 – 11.66) | 0.291 |
| No | 90 (18.37) | 400 (81.63) | 1 | 117 (15.18) | 654 (84.82) | 1 | |||
| > 75 years | Yes | 2 (0.69) | 286 (99.31) | 0.48 (0.09 – 2.34) | 0.497 | 0 (0.00) | 7 (100.00) | 1.000 | |
| No | 7 (1.43) | 483 (98.57) | 1 | 9 (1.17) | 762 (98.83) | 1 | |||
Table 5: Distribution of patients by age groups and the Schistosoma haematobium and Schistosoma mansoni infection positivity of the rectal mucosal biopsy
| Clinical Indications | Schistosoma Haematobium | Schistosoma Mansoni | ||||||
|---|---|---|---|---|---|---|---|---|
| Positive, n (%) | Negative, n (%) | Crude OR (95% CI) | p-value | Positive, n (%) | Negative, n (%) | Crude OR (95% CI) | p-value | |
| Hematuria (yes) | 36 (12.46) | 35 (6.97) | 1.89 (1.16 – 3.09) | 0.009 | 1 (14.29) | 70 (8.93) | 1.70 (0.20 – 14.32) | 0.484 |
| 253 (87.54) | 467 (93.03) | 6 (85.71) | 714 (91.07) | 1 | ||||
| Rectal bleeding (yes) | 3 (1.04) | 13 (2.59) | 0.39 (0.11 – 1.39) | 0.136 | 0 (0.00) | 16 (2.04) | 1.000 | |
| 286 (98.96) | 489 (97.41) | 7 (100.00) | 768 (97.94) | |||||
| Edematous syndrome (yes) | 31 (10.73) | 56 (11.16) | 0.96 (0.60 – 1.52) | 0.852 | 1 (14.29) | 86 (10.97) | 1.35 (0.16 – 11.37) | 0.559 |
| 258 (89.27) | 446 (88.84) | 6 (85.71) | 698 (89.03) | |||||
| Hepatomegaly (yes) | 1 (0.35) | 2 (0.40) | 0.87 (0.01 – 9.62) | 1.000 | 0 (0.00) | 3 (0.38) | 1.000 | |
| 288 (99.65) | 500 (99.60) | 7 (100.00) | 781 (99.62) | |||||
| Splenomegaly (yes) | 14 (4.84) | 37 (7.37) | 0.64 (0.34 – 1.20) | 0.164 | 2 (28.57) | 49 (6.25) | 6.00 (1.14 – 31.72) | 0.069 |
| 275 (95.16) | 465 (92.63) | 5 (71.43) | 735 (93.75) | |||||
| Hepatosplenomegaly (yes) | 4 (1.38) | 14 (2.79) | 0.49 (0.16 – 1.50) | 0.202 | 1 (14.29) | 17 (2.17) | 7.52 (0.86 – 65.91) | 0.149 |
| 285 (98.62) | 488 (97.21) | 6 (85.71) | 767 (97.83) | |||||
| Schistosomiasis related symptoms (yes) | 150 (51.90) | 236 (47.01) | 1.22 (0.91 – 1.63) | 0.185 | 0 (0.00) | 386 (49.23) | 0.015 | |
| 139 (48.10) | 266 (52.99) | 7 (100.00) | 398 (50.77) | |||||
| Portal hypertension syndrome (yes) | 0 (0.00) | 3 (0.60) | – | 0.558 | 0 (0.00) | 3 (0.38) | 1.000 | |
| 289 (100.00) | 499 (99.40) | 7 (100.00) | 781 (99.62) | |||||
| Anemic syndrome (yes) | 1 (0.35) | 7 (1.39) | 0.25 (0.03 – 2.00) | 0.269 | 0 (0.00) | 8 (1.02) | 1.000 | |
| 288 (99.65) | 495 (98.61) | 7 (100.00) | 776 (98.98) | |||||
| Others (yes) | 49 (16.96) | 99 (19.72) | 0.83 (0.57 – 1.21) | 0.337 | 2 (28.57) | 146 (18.62) | 1.75 (0.33 – 9.09) | 0.621 |
| 240 (83.04) | 403 (80.28) | 5 (71.43) | 638 (81.38) | |||||
Table 6: Distribution of patients according to the clinical indications and the Schistosoma haematobium and Schistosoma mansoni infection positivity of the rectal mucosal biopsy
| Variablesa | Positive, n (%) | Negative, n (%) | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Ethnic Group | ||||||
| Malinké | 26 (8.50) | 28 (5.24) | 1.68 (0.96 – 2.91) | 0.064 | 1.89 (1.06 – 3.38) | 0.031 |
| Soninké | 32 (10.46) | 29 (5.43) | 2.03 (1.20 – 3.43) | 0.006 | 1.89 (1.09 – 3.27) | 0.023 |
| Profession | ||||||
| Pupil/Student | 93 (30.39) | 117 (21.91) | 1.56 (1.13 – 2.14) | 0.006 | 1.69 (1.19 – 2.40) | 0.003 |
| Trader | 38 (12.42) | 30 (5.62) | 2.38 (1.44 – 3.93) | 0.001 | 2.42 (1.43 – 4.12) | 0.001 |
| Civil servant | 13 (4.25) | 44 (8.24) | 0.49 (0.26 – 0.93) | 0.027 | ||
| Residence | ||||||
| Sikasso | 12 (3.92) | 55 (10.30) | 0.36 (0.18 – 0.67) | 0.001 | 0.45 (0.22 – 0.87) | 0.017 |
| Ségou | 45 (14.71) | 35 (6.55) | 2.46 (1.54 – 3.92) | 0.000 | 2.66 (1.63 – 4.87) | 0.000 |
| Mopti | 20 (6.54) | 19 (3.56) | 1.89 (0.99 – 3.61) | 0.048 | 2.04 (1.04 – 4.00) | 0.039 |
| Gao | 3 (0.98) | 12 (3.56) | 0.43 (0.12 – 1.54) | 0.182 | ||
| Sex | ||||||
| Male | 214 (39.41) | 69 (31.08) | 1.44 (1.03 – 2.01) | 0.030 | ||
| Age Group | ||||||
| < 24 years | 130 (45.14) | 193 (39.39) | 1.26 (0.94 – 1.69) | 0.116 | ||
| 50 – 74 years | 29 (10.07) | 90 (18.37) | 0.49 (0.31 – 0.78) | 0.002 | 0.60 (0.37 – 0.97) | 0.035 |
| Clinical Indications | ||||||
| Hematuria | 36 (12.46) | 35 (6.97) | 1.90 (1.16 – 3.09) | 0.009 | 1.78 (1.06 – 2.99) | 0.029 |
| Rectal bleeding | 3 (1.04) | 13 (2.59) | 0.39 (0.11 – 1.40) | 0.136 | ||
| Splenomegaly | 14 (4.84) | 37 (7.37) | 0.64 (0.34 – 1.21) | 0.164 | ||
| Schistosomiasis-related symptomsb | 150 (51.90) | 236 (47.01) | 1.21 (0.92 – 1.61) | 0.185 | ||
| Year of Biopsy | ||||||
| 2011 | 101 (33.01) | 151 (28.28) | 1.25 (0.92 – 1.69) | 0.150 | ||
| 2012 | 74 (24.18) | 108 (20.22) | 1.26 (0.89 – 1.76) | 0.180 | ||
| 2013 | 53 (17.32) | 120 (22.47) | 0.72 (0.50 – 1.04) | 0.076 | 0.72 (0.49 – 1.05) | 0.089 |
| 2014 | 23 (7.52) | 60 (11.24) | 0.64 (0.39 – 1.06) | 0.082 | 0.64 (0.38 – 1.08) | 0.096 |
| 2017 | 3 (0.98) | 19 (3.56) | 0.26 (0.07 – 0.91) | 0.024 | 0.26 (0.07 – 0.90) | 0.035 |
a Variables with p-value < 0.20 included in model.
b Schistosomiasis-related symptoms: infectious work-up, and suspicion of schistosomiasis.
Table 7: Results of multivariate logistic regression for the factors associated with Schistosoma haematobium infection positivity of the rectal mucosal biopsy
Figures








Keywords
- Neglected tropical diseases
- Schistosomiasis
- Parasitic diseases
- Epidemiology
- Internal medicine
