Systematic Review | Open Access | Volume 8 (3): Article 74 | Published: 16 Sep 2025
Effectiveness of malaria control interventions in resource-limited countries: A systematic review
Menu, Tables and Figures
Navigate this article
Tables
Table 1: Quality ratings for study type and key outcomes using GRADE
| Study Design | Number of Studies | Initial Quality | Upgrades/Downgrades | Final Quality | Reference |
|---|---|---|---|---|---|
| Cross-sectional study | 3 | Low | Downgraded for risk of bias (due to observational nature), imprecision (small sample sizes) | Low | [20], [27], [15] |
| Laboratory-based experimental study | 1 | High | No downgrade (precise, well-conducted experimental study) | High | [17] |
| Mathematical model study | 1 | Moderate | Downgraded for indirectness (models may not fully reflect real-world complexity) | Low | [23] |
| Mixed-method study | 1 | Low | Upgraded for consistency and directness (results align with other intervention studies) | Moderate | [19] |
| Qualitative study | 1 | Low | Downgraded for imprecision and indirectness (limited generalizability, small sample) | Low | [14] |
| Before-and-after study | 1 | Moderate | Downgraded for bias and potential confounding (no control group) | Low | [22] |
| Retrospective cohort study | 1 | Moderate | Downgraded for bias (observational nature, potential confounding) | Low | [21] |
| Retrospective observational study | 2 | Moderate | Downgraded for imprecision (small sample sizes, retrospective nature introduces bias) | Low | [24], [13] |
| Retrospective study | 2 | Moderate | Downgraded for risk of bias (potential confounding, lack of prospective data) | Low | [18], [26] |
| Theoretical modeling study | 1 | Moderate | Downgraded for indirectness and imprecision (model assumptions, small case study applications) | Low | [25] |
| Randomized controlled trial (RCT) | 1 | High | No downgrade (randomization and blinding ensured high methodological rigor) | High | [16] |
Table 1: Quality ratings for study type and key outcomes using GRADE
Table 2: Pooled Effect Size and Heterogeneity Statistics
| Parameter | Estimate | 95% Confidence Interval | p-value |
|---|---|---|---|
| Effect Size (dˉ) | 1.27 | [0.58, 1.96] | < .001 |
| Heterogeneity Statistics | |||
| – Q (Residual Heterogeneity) | 105.97 | df = 8 | < .001 |
| – τ² (Between-Study Variance) | 1.06 | ||
| – τ (Standard Deviation) | 1.03 | ||
| – I² (%) | 98.08 | ||
| – H² | 52.17 | ||
Table 2: Pooled Effect Size and Heterogeneity Statistics
Figures


Keywords
- Effectiveness
- Malaria
- Control
- Interventions
- Resource-limited
- Countries
Emmanuel George Bachan1,2, Juliet Abu3, Martin Nyaaba Adokiya4, Charles Tobin-West2
1Bono Regional Health Directorate, Ghana Health Service, Sunyani, Ghana, 2School of Public Health, University of Port Harcourt, Port Harcourt, Nigeria, 3College of Nursing and Midwifery, Sunyani, Ghana, 4University for Development Studies (UDS), Tamale, Ghana
&Corresponding author: Emmanuel George Bachan, Ghana Health Service, Sunyani, Ghana, Email: bachangeorge@yahoo.com, ORCID: https://orcid.org/0000-0003-0116-5723
Received: 05 Mar 2025, Accepted: 15 Sep 2025, Published: 16 Sep 2025
Domain: Infectious Disease Epidemiology, Malaria Control
Keywords: Effectiveness, Malaria, Control, interventions, Resource-limited, Countries
©Emmanuel George Bachan et al. Journal of Interventional Epidemiology and Public Health (ISSN: 2664-2824). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Emmanuel George Bachan et al., Effectiveness of malaria control interventions in resource-limited countries: A systematic review. Journal of Interventional Epidemiology and Public Health. 2025;8(3):74. https://doi.org/10.37432/jieph-d-25-00060
Abstract
Introduction: Malaria remains a key public health encounter in countries with resource limitations, where efforts to control the disease often focus on vector control strategies like insecticide-treated nets (ITNs) and indoor residual spraying (IRS), but its global burden remains high. Integrated malaria prevention, combining multiple interventions, has shown promise in reducing malaria incidence, though challenges such as high costs and insecticide resistance limit its widespread implementation in these countries. We assessed the effectiveness of malaria control interventions among individuals aged five years and above in resource-limited countries.
Methods: We used a wide-range search strategy to identify studies from reputable databases, including PubMed, Google Scholar, Cochrane Library, African Journal Online, and Science Direct, using keywords related to the effectiveness of malaria control interventions. The inclusion criteria focused on peer-reviewed articles published between January 2018 and September 2024, excluding systematic reviews. The selection process was guided by PRISMA 2020 guidelines, with multiple reviewers independently extracting data on the effectiveness of malaria control interventions. The findings were synthesised quantitatively and qualitatively. The study’s quality was assessed using the GRADE framework and critically appraised using Bradford Hill’s criteria.
Results: Fifteen studies met the inclusion criteria, encompassing diverse geographical regions and study designs, including cross-sectional, before-and-after and RCT. The study found that individual interventions, such as IRS and ITNs, had varying effectiveness, with IRS using non-pyrethroid insecticides being highly effective. Larviciding had a small negative effect, and Intermittent Preventive Treatment was moderately effective in pregnant women.
Conclusion: The evidence is limited by high heterogeneity in studies, focusing mainly on Sub-Saharan Africa. Integrated malaria control strategies, including ITNs and IRS, are effective in resource-limited settings. Prioritizing combined interventions and non-pyrethroid IRS is crucial. However, challenges such as insecticide resistance and the need for sustained interventions remain. Future research should focus on long-term effectiveness, including the use of natural products, larviciding and other supplementary strategies for vector control. Continuous research on climate impacts, new technologies, and emerging threats is key to achieving effective and sustainable malaria control.
Introduction
Malaria remains a noteworthy public health burden, particularly in resource-limited countries, where it contributes to high morbidity and mortality rates. In 2023, there were an estimated 263 million malaria cases globally, with 200 million of these attributable to the WHO African Region and 180 million of these to Sub-Saharan Africa. The total number of global cases stood at 120 million in people five years and older, 90 million in the WHO African Region and 80 million in Sub-Saharan Africa [1].
Efforts to combat malaria in resource-limited countries have primarily focused on vector control strategies, involving the use of ITNs and IRS. These interventions have been widely implemented and have shown effectiveness in reducing malaria transmission [2]. However, the malaria global burden in current years has not reduced significantly, highlighting urgent need for more comprehensive approaches [2]. Integrated malaria prevention, which involves the use of multiple interventions simultaneously, has been proposed as a more effective strategy[3]. This approach includes not only ITNs and IRS but also other measures such as larval source management, limitation of mosquito entry via quality housing and use of topical repellents[4]. Studies have shown that combining multiple interventions can significantly reduce malaria incidence and prevalence compared to single-method approaches [4].
Despite the potential benefits, the implementation of integrated malaria prevention strategies faces several challenges, including high costs, labor intensity, and potential side effects of insecticide-based methods[5]. Moreover, there is limited evidence synthesizing the effectiveness of these combined interventions in resource-limited settings[5].
Several factors influence the effectiveness of malaria control in resource-limited countries. Socio-economic status plays a critical role, as poverty limits access to preventive measures like ITNs and treatments, making it harder to control malaria. Health system capacity is another key factor, as weak health systems may lack the resources and infrastructure needed to implement and sustain effective malaria control programs. Environmental conditions, such as proximity to water bodies and climatic factors, can also significantly impact mosquito breeding and malaria transmission, complicating efforts to reduce the disease’s spread.
Additionally, insecticide resistance poses a serious challenge, as mosquitoes develop resistance to insecticides applied in ITNs and IRS, lowering the success of these interventions[6]. Community engagement is equally important, as successful malaria control programs often depend on the active participation of local populations in adopting preventive measures and seeking treatment [7]. Without sufficient community involvement, even the most well-designed malaria control strategies may fail to achieve their intended impact.
Resource-limited countries employ a variety of malaria control interventions, including ITNs, IRS, larval source management (LSM), artemisinin-based combination therapies (ACTs), intermittent preventive treatment (IPT), rapid diagnostic tests (RDTs), seasonal malaria chemoprevention (SMC), health education and community engagement. These interventions aim to prevent mosquito bites, reduce mosquito populations, ensure early diagnosis and treatment, and raise awareness about malaria prevention practices [8].
Several reviews and studies have been conducted on malaria control interventions. These reviews typically focus on the success of various interventions, the epidemiology of malaria, and the socio-economic factors influencing malaria transmission and control. These studies examined the effectiveness of various malaria interventions, including bednet usage, indoor residual spraying, and health system improvements [8][9]. From these reviews there was little focus on intervention that benefits persons 5years and above.
We assessed the effectiveness of malaria control interventions among individuals aged five years and above in resource-limited countries. This review stands out from previous ones[10], [11]. It has a wider range of malaria control interventions that specifically targeted persons aged five years and above. It discusses the integration of various control measures, including larviciding, which is often underrepresented in other reviews. Methodologically, it adopts a more comprehensive approach, utilizing an extensive literature search, refined inclusion criteria, and used Bradford Hill’s criteria for critical appraisal. Lastly, it incorporated more recent studies and data from January 2018 to September 2024, offering an updated and current perspective on effectiveness of malaria control interventions.
This review is expected to make significant contributions to both knowledge and practice in the malaria control intervention’s effectiveness and practically inform policymakers with evidence-based recommendations, aid in the formulation of effective malaria control policies. It provides valuable insights for health workers and community organizations on scaling up malaria control interventions.
Methods
Study Design
This systematic review evaluated various malaria control interventions targeting persons aged five years and above across different resource-limited countries. The review identified and explored the effectiveness of malaria control interventions, including insecticide-treated nets (ITNs), long-lasting insecticidal nets (LLINs), indoor residual spraying (IRS), chemoprevention, antimalarial drugs, and more in different settings.
Information Sources
To gather relevant studies, a wide range search was done using several reputable databases and online platforms, including PubMed, Google Scholar, Cochrane Library, African Journal Online, Science Direct and Web of Science. These databases were selected based on their extensive coverage of health-related literature and their relevance to malaria control research.
Search Strategy
The search involved a broad set of keywords and terms to identify studies related to malaria control interventions. Key search terms included malaria prevention, treatment, management, eradication, and elimination.
Challenges of specific interventions such as ITNs, long-lasting insecticidal nets (LLINs), IRS, chemoprevention, artemisinin-based combination therapies (ACTs), seasonal malaria chemoprevention (SMC), IPT, vaccination, larviciding, environmental management, and community case management were searched.
The search terms were linked with Boolean operators such as “AND” and “OR” to improve the search results. The review also specified age groups (e.g., individuals aged 5 years and above, children over 5 years, adolescents, adults, and the elderly) and geographic regions, focusing on low- and middle-income countries (LMIC), predominantly sub-Saharan Africa.
Eligibility criteria
Inclusion Criteria: Studies focusing on any malaria control interventions targeting persons five years and above, studies conducted in resource-limited countries, peer-reviewed articles, studies published between January 2018 and September 2024 and articles available in English were included.
Exclusion Criteria: Papers that had only abstracts were excluded.
Study Selection Process
The selection process followed the PRISMA guidelines, starting with the identification of studies through database searches, followed by screening titles and abstracts. Articles were then checked for eligibility based on the inclusion and exclusion criteria. The review process was iterative, ensuring that all relevant studies were included while duplicates and irrelevant articles were excluded.
Data Items
The selected studies were reviewed independently by multiple reviewers, and discrepancies were resolved through discussion and consensus. Data extraction focused on key elements, such as the study area, study design, malaria intervention type, outcomes (malaria incidence, prevalence, mortality rates, morbidity rates, cost-effectiveness). The data were organized by region and study design to identify trends and patterns.
Data Synthesis
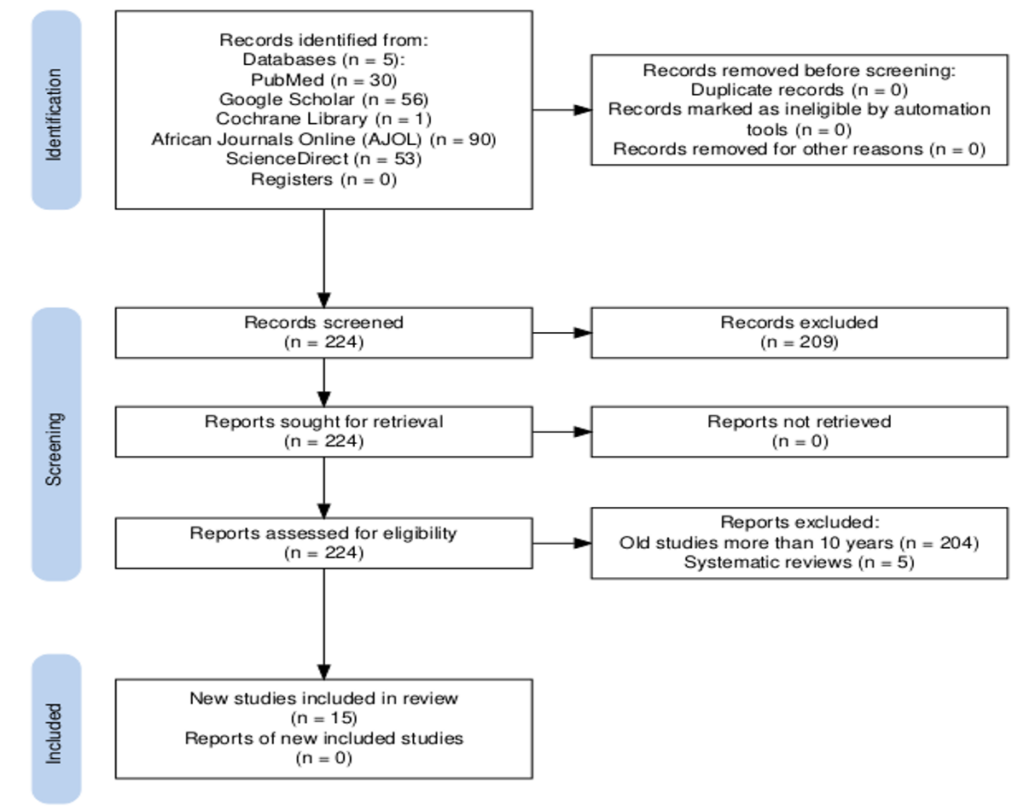
The review synthesized findings qualitatively, summarizing key results from the studies across different geographical regions and study designs. In addition, meta-analysis was conducted; we collectively analyzed the diversity of study design and outcomes (Odd ratio, incidence, prevalence) while taking into account heterogeneity given the wide variety of methodology and population. . The study systematically selected 15 studies for synthesis through a three-step process: dual-reviewer screening, independent full-text review, data extraction, and tabulation. All studies were analyzed by theme and region, with 6 studies grouped by intervention type for meta-analysis. Narrative synthesis was used for all studies, and a random-effects meta-analysis was conducted for a subset of six studies. Results were displayed using PRISMA flowcharts and forest plots.
Ethical Considerations
This review involved secondary data analysis of publicly available studies and did not require ethical approval. However, ethical standards were adhered to by only including studies published in peer-reviewed journals.
Quality Assessment
The quality of the studies included was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework (Table 1)
Results
Current status of knowledge
The review included a total of 15 studies out of 224 studies screened. Of these studies, three were from Ghana, three from Tanzania, two from Nigeria and one each from Botswana, Mali, Democratic Republic of Congo (DRC), Ethiopia, Kenya, across Sub-Saharan Africa, and Myanmar
In Ghana, recent research highlighted the significance of integrated malaria prevention measures, noting that malaria prevalence was dramatically decreased by having access to IRS and ITNs, with the integrated use of both treatments proving especially successful [12]. Household access to ITNs contributed to a 7.1% point reduction in malaria prevalence, IRS contributed to a 6.8% point reduction, while ITNs and IRS combination led to a 27.1% point decrease. Meanwhile, IRS significantly decreased malaria cases in Northern, Upper East and West regions of Ghana with 39-58% fewer cases in IRS districts [13]. Nevertheless, its removal caused an increase in incidence, emphasizing the need for ongoing interventions. Furthermore, despite implementation issues, Mass Test, Treat, and Track (MTTT) programs were perceived to effectively reduce Pakro sub-district malaria incidence [14]. These results highlight the necessity of ongoing and coordinated efforts to control malaria in Ghana to have a long-lasting effect.
In Nigeria, recent studies have highlighted innovative approaches to malaria prevention. The use of LLINs significantly increased the acceptance of IPT among pregnant women in Ibadan, their use was related with acceptance of IPTsp (p<0.05) suggesting that LLINs can enhance the effectiveness of IPT programs[15]. Meanwhile, neem seed oil cream demonstrated that it is an effective natural repellent against Anopheles gambiae mosquitoes, with higher concentrations providing longer protection [16]. That is the 10.0% w/w (weight/weight) concentration of neem seed oil cream provided the longest duration of protection and outperformed the commercial Deet repellent. These findings underscore the potential of integrating LLINs with IPT programs and utilizing natural repellents like neem seed oil to improve malaria prevention strategies in Nigeria.
In Tanzania, recent research demonstrated that Dioscorea sansibarensis leaf extract possesses significant larvicidal potency, suggesting its potential as a natural vector control agent.The LC50 values for Cx. quinquefasciatus was between 60.915 to 152.944 ppm and An. gambiae s.s., 80.700 to 169.659 ppm indicating a high larvicidal potency of the extract [17]. In another study, it was found out that combining window screening with larviciding in urban Dar es Salaam significantly reduced both malaria prevalence and mosquito densities by 50%, showcasing the effectiveness of integrated approaches [18]. Additionally, a study reported high community acceptance (80.3%) of biolarviciding in Southern Tanzania, driven by perceptions of its effectiveness despite limited knowledge [19]. These studies underscore the importance of using natural products, integrated interventions and community engagement in enhancing malaria control efforts in Tanzania.
A cross-sectional study conducted in DRC, demonstrated that there was no substantial difference in malaria transmission rates after ITNs distribution. The mosquitoes were resistant to pyrethroids and DDT but inclined to the carbamate insecticide bendiocarb. Resistance to pyrethroids was at least partially due to metabolic resistance mechanisms, as the addition of a synergist increased mosquito mortality. The frequency of the kdr West resistance mutation increased from 92% to 99% after the ITN distribution [20].
In Botswana’s Okavango Delta, adaptive strategies (combining health information/education meetings (69%), modifying house buildings (49.4%), and timing activities/limiting movement (43%) by households significantly improved malaria control[21]. This underscores the importance of flexible approaches in combating the disease.
In Ethiopia, indoor residual spraying and insecticide-treated mosquito nets interventions successfully reduced mean malaria cases after the implementation of strategic interventions in most regions. Malaria cases across different regions varied significantly from a 9.19% reduction to a 69.19% reduction when compared to what would have been expected without the intervention. Mean malaria-related death in all regions decline rate was 37.93%, minimum region and 90.43% maximum showcasing the effectiveness of these measures [22].
In Kenya, combining social media campaigns with other control measures such LLIN and IRS further enhance malaria control efforts [23]. Meanwhile, the study in Mali’s Ségou region, observed rapid reductions in malaria incidence following IRS where IRS districts had a projected 286,745 fewer overall cases of malaria and suspension of IRS in one district led to a 70% rise in malaria incidence [24]. The studies in Kenya, and Mali collectively highlight the diverse and context-specific strategies that can be employed to combat malaria across different regions.
A Sub-Saharan Africa study emphasized the effectiveness of longer-lasting ITNs through theoretical modeling, which also highlighted the necessity for adaptive strategies such as regular and early replacement of ITNs, increased ITN Coverage, education on proper ITN use and care, targeting temperature-sensitive parameters to address temperature variations that can trigger malaria outbreaks [25]. Meanwhile, a study done in Eastern Myanmar, found that improved data transmission and reporting were crucial in reducing malaria incidence by providing the critical information needed to make informed, rapid decisions about where to deploy limited resources for maximum impact, underscoring the importance of effective data management in disease control efforts [26]. These findings illustrate the critical role of both innovative tools and robust data systems in enhancing malaria control across diverse regions.
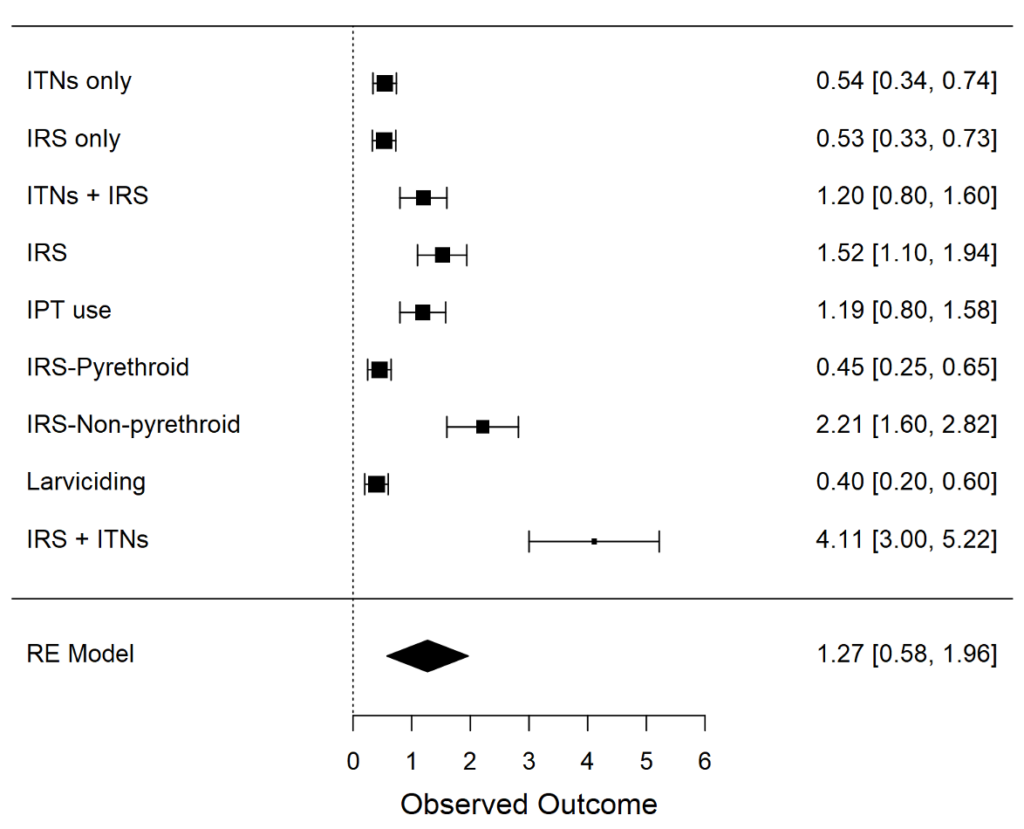
The meta-analysis pooled data from 6 studies of malaria control interventions on insecticide-treated nets (ITNs), indoor residual spraying (IRS), intermittent preventive treatment (IPT), larviciding, and combinations of these. The pooled generalized effect size (dˉ=1.27, 95% CI: [0.58, 1.96]) shows a moderate to large magnitude of effect on overall malaria prevalence or incidence by these interventions (Cohen’s conventions suggest d=0.2 is small, d=0.5 is medium, and d=0.8 is large). This finding is statistically significant (p<. 001), indicating the general effectiveness of these strategies in preventing malaria.
Moderate effects are seen due to indoor residual spraying (IRS) (d=1.52). It appears that IRS with non-pyrethroid (d=2.21) is more effective than ITN only, indicating that this method could be considered by many in certain contexts where there are challenges surrounding pyrethroid resistance.
Larviciding (d=−0.40) has a small but negative effect, which may be explained by implementation challenges, limited scope, or contextual factors that reduce its effectiveness.
ITNs + IRS (large effect size d =4.11), highlighting the added benefit of integrating a portfolio of strategies to derive greater impact. This implies the necessity of using combination strategies to maximize malaria prevention strategies.
Although IPT use in pregnant mothers shows moderate effectiveness (d=1.19) [46]; yet supports targeted malaria prevention for high-risk populations. This finding further underscores the importance of prioritizing IPT in maternal health programs.
The analysis indicates significant study heterogeneity (I2=98.08%), which may be influenced by differences in the type of intervention, countries, populations or measurement approaches. Such variability highlights both the complexity of malaria control and the necessity for context-specific approaches.
Discussion
Using Bradford Hill’s nine criteria, all reviewed studies on the effectiveness of malaria interventions were critically appraised to find out how the findings align with each criterion.
Strength of association
The strength of association between various malaria control interventions and their effectiveness varied significantly across different studies. In Ghana, a study demonstrated a substantial reduction in malaria prevalence by 27.1 percentage points when combining ITN and IRS [27]. This indicated a strong association with the effectiveness of these interventions. Similarly, a 39-58% reduction in malaria cases due to IRS reported in Ghana reflected a strong association with reduced malaria incidence[13]. In Mali, a rapid reduction in malaria incidence following IRS implementation and an increase after its suspension was observed[24] which underscored the strong association with IRS effectiveness.
On the other hand, studies showing a moderate association included the one in Sub-Saharan Africa, which highlighted the effectiveness of longer-lasting ITNs and the effect of temperature changes on malaria control [25]. This study emphasized the importance of adaptive strategies. A study in Tanzania also found a moderate association, noting reductions in malaria prevalence and mosquito densities due to integrated approaches like window screening and larviciding [18].
Conversely, the DRC study found a weak association, as there was no significant reduction in malaria transmission despite ITN distribution [20]. This weak association was primarily attributed to high insecticide resistance and highlights the challenges faced in certain regions. These varied degrees of association underscored the intricacies of malaria control and the need for tailored strategies to address specific local challenges.
Consistency
Two studies in Ghana provides consistent evidence that vector control interventions, such as ITNs and IRS, were effective in reducing malaria prevalence studies [12], [13]. Both studies highlighted the significant impact of these interventions, reinforcing their reliability and effectiveness in malaria control. Similarly, a study in Tanzania found consistent results across various settings and demonstrated that integrated approaches, including window screening and larviciding, effectively reduced malaria prevalence [18]. These consistent findings across different regions and methodologies underscored the robustness of these interventions in combating malaria.
In contrast, the study in the DRC presents inconsistent findings compared to other studies [20]. The lack of significant reduction in malaria transmission despite ITN distribution is primarily attributed to high levels of insecticide resistance. This inconsistency highlighted the challenges posed by resistance issues, which can undermine the effectiveness of otherwise proven interventions.
Specificity
The study in Ghana provides clear evidence of the specific effects of IRS on malaria reduction [24]. The significant decrease in malaria cases directly attributed to IRS implementation underscored the targeted impact of this intervention. This specificity is crucial as it highlighted the direct correlation between the intervention and the observed health outcomes, reinforcing the effectiveness of IRS in malaria control.
Similarly, the Tanzania study demonstrates the specific larvicidal effects of Dioscorea sansibarensis on malaria vectors[17]. This study emphasized the targeted impact of this natural larvicide, which specifically affects the mosquito larvae responsible for malaria transmission. The specificity of this effect is important as it provided a focused approach to reducing the vector population, thereby contributing to malaria control efforts.
In contrast, the study in Botswana illustrates the less specific effects of adaptive strategies on malaria control [21]. While these strategies had positive impacts, they were not exclusively aimed at malaria prevention. This lack of specificity meant that the observed benefits could be attributed to a range of factors, not solely to malaria control interventions. The broader scope of these adaptive strategies, while beneficial, made it challenging to pinpoint their direct impact on malaria prevention. There is therefore the need for focused strategies to effectively combat malaria.
Temporality
In the study conducted in Mali, clear temporality is evident. The rapid decline in malaria cases after the implementation of IRS and the subsequent increase after its withdrawal provide a strong temporal association [24]. This pattern suggests the intervention had a direct impact on reducing malaria cases, and its removal led to a resurgence, highlighting the importance of continuous intervention to maintain low incidence rates.
Similarly, research in Sub-Saharan Africa showcased clear temporality through the impact of ITNs and temperature variations over time [25]. The study demonstrated how the introduction of more durable ITNs led to a sustained decline in malaria transmission. Additionally, the analysis of temperature variations over time and their correlation with malaria incidence further supported the temporal relationship, an indication that both interventions and environmental factors played significant roles in malaria control.
On the other hand, the study in the DRC presented less clear temporality. Despite the distribution of ITNs, there was a lack of significant impact on malaria incidence [20]. This ambiguity in temporality may be attributed to issues such as insecticide resistance, which can undermine the effectiveness of ITNs. These studies underscored the need for continuous monitoring and adaptation of malaria control strategies to ensure their sustained effectiveness.
Biological gradient, or dose-response
In the study conducted in Nigeria, clear evidence of a dose-response relationship was observed with the use of neem seed oil cream[16]. The study found that higher concentrations of the cream provided longer protection against malaria-carrying mosquitoes. This gradient indicates that the effectiveness of the neem seed oil cream is directly proportional to its concentration, thereby supported the concept of a biological gradient. The higher the dose of the active ingredient, the greater the protective effect, which is a strong indicator of the intervention’s efficacy.
Similarly, the Ghanaian study demonstrated a dose-response relationship through the combined use of ITNs and IRS [12]. The study showed that the combination of these two interventions led to a greater reduction in malaria prevalence compared to the use of either intervention alone.
Conversely, the study in the DRC presents less evidence of a biological gradient. Despite the high coverage of ITNs, the impact on malaria incidence was minimal[20]. This lack of a clear dose-response relationship can be attributed to high levels of insecticide resistance among the mosquito population. Resistance undermines the effectiveness of ITNs, meaning that even with widespread use, the expected reduction in malaria cases was not observed.
Plausibility
In the study conducted in Tanzania, the larvicidal potency of Dioscorea sansibarensis is considered biologically plausible. This plant targeted malaria vectors directly by affecting the larvae of mosquitoes, which are the primary carriers of the malaria parasite [17]. The mechanism was straightforward since by reducing the mosquito population at the larval stage, the overall transmission of malaria decreased. This direct targeting of vectors provided a clear and logical pathway for how the intervention can effectively reduce malaria incidence, making the mechanism highly plausible.
Similarly, in Tanzania a study explored integrated control measures such as window screening and larviciding. These measures aligned well with biological mechanisms aimed at reducing mosquito populations and, consequently, malaria transmission. The biological plausibility of these integrated measures is supported by their alignment with known mechanisms of vector control.
On the other hand, the study in the DRC presents less plausible mechanisms [20]. The high levels of insecticide resistance among mosquito populations made the effectiveness of ITNs less plausible as a sole intervention strategy. When mosquitoes develop resistance to the insecticides used in ITNs, the expected reduction in malaria transmission is not achieved. This resistance undermines the biological mechanism that ITNs rely on, which is to kill or repel mosquitoes upon contact. As a result, the intervention’s effectiveness was compromised, making the mechanism less plausible in the context of high resistance.
Coherence
The studies in Ghana provided coherent findings that align with the established understanding that combining vector control interventions leads to significant reductions in malaria. Both studies demonstrated that the use of multiple interventions, such as ITNs and IRS, resulted in a greater decrease in malaria prevalence compared to using a single method [12], [13]. This coherence with existing knowledge supports the idea that a multi-faceted approach is more effective in controlling malaria, as it addresses different aspects of mosquito behavior and lifecycle, thereby reducing the overall transmission of the disease.
Similarly, in Tanzania it was found that the integration of various control measures, such as window screening and larviciding, is coherent with the broader understanding of multi-faceted approaches to malaria control [18]. The study’s findings aligned with the theory that combining different strategies can provide a more comprehensive and effective means of reducing mosquito populations and malaria transmission. This coherence reinforces the importance of integrated vector management, which is widely recognized as a best practice in malaria control.
On the other hand, the study in the DRC presents less coherent findings [20]. Despite the distribution of ITNs, there was no significant reduction in malaria transmission. This outcome is less coherent with the general expectations of ITN effectiveness, which typically showed a decrease in malaria cases following widespread ITN use.
Experiments
In the context of mosquito control, several studies have provided supportive experimental evidence for the effectiveness of natural agents. For instance, the laboratory-based experiments conducted in Tanzania demonstrated the larvicidal properties of Dioscorea sansibarensis. This study highlighted the potential of this plant as a natural alternative to chemical larvicides, which are often associated with environmental and health concerns.
Similarly, a study in Nigeria provided experimental evidence supporting the use of neem seed oil as a mosquito repellent [16]. Their findings indicated that neem seed oil can effectively repel mosquitoes, offering a natural and potentially safer alternative to synthetic repellents. This is particularly significant in regions where mosquito-borne diseases are prevalent, and there is a need for accessible and affordable mosquito control methods.
The supportive experimental evidence from [17] and [16]underscored the potential of natural agents in mosquito control. These studies provide a foundation for further research and development of environmentally friendly and sustainable mosquito control methods. The effectiveness of Dioscorea sansibarensis as a larvicidal agent and neem seed oil as a mosquito repellent could lead to the development of new products that are both effective and safe for human use.
In contrast, the limited experimental evidence from [20] highlighted the need for more comprehensive and robust experimental designs. Addressing resistance mechanisms is crucial for the long-term success of ITNs and other mosquito control strategies. Future research should focus on understanding and mitigating resistance to ensure the continued effectiveness of these interventions.
Analogy
In the context of malaria control, several studies demonstrated analogous findings, reinforcing the combined effectiveness of ITNs and IRS. Studies in Ghana found that the combined use of ITNs and IRS significantly reduced malaria incidence [17], [16]. These findings were consistent with other studies that have shown the synergistic effects of using multiple interventions to combat malaria.
Similarly, a study in Tanzania highlighted the effectiveness of integrated approaches in malaria control [18]. Their findings aligned with similar studies from other regions, demonstrating that combining various strategies, such as ITNs, IRS, and environmental management, can lead to substantial reductions in malaria transmission. This integrated approach is crucial in areas with high malaria transmission, as it addresses multiple aspects of the mosquito life cycle and habitat.
Community engagement enhances malaria control by building trust, embedding strategies, and empowering communities through education and participation, building resilient systems and addressing implementation hurdles in Tanzania, Nigeria, and Botswana.
Data is crucial for drug-resistant malaria control, enabling surveillance, intervention evaluation, and predictive modeling. It guides actions against emerging threats in DRC, Mali, and Myanmar, facilitating timely responses, targeted resource allocation, and adaptation to changing environmental and epidemiological conditions.
We recognize the narrow scope of the review may hinder the generalizability of findings to other LMICs. Future research should also expand the geographical scope to areas such as South Asia, Latin America and the Caribbean for enhanced robustness and applicability. In addition, improving the quality of future research in this area is key.
The key findings from the meta-analysis suggested that both ITNs (d=0.54) and IRS (d=1.52) produce moderate-to-large effects, confirming their role as independent tools for malaria prevention. In particular, IRS using non-pyrethroid (d=2.21) is highly efficacious; it thus represents a promising tool for those settings that are currently struggling with pyrethroid resistance.
Although Larviciding (d=−0.40) had a limited effect, this may be due to challenges in implementation and coverage, which warrant further research to optimize its effectiveness. Future research is needed to evaluate multi-intervention vector control strategies that include larviciding, particularly in combination with LLINs and/or IRS, due to current evidence gaps in their effectiveness for malaria control.
The dual impact of combining ITNs with IRS (d=4.11) is affording it some of the largest margins of effect sizes confirming the benefit of multimodal strategies to maximize delivery. This favors the implementation of combined strategies in areas endemic for malaria where these resources are available.
Pregnant women have moderate effectiveness of IPT use (d=1.19), suggesting its role in protection of high-risk population. This highlights the need to integrate IPT into maternal health programmes to protect pregnant women and their foetuses.
The heterogeneity in studies suggests a need for context-specific approaches, prioritizing non-pyrethroid IRS and combined interventions for malaria control due to their greater effectiveness.
ITNs and IRS are cost-effective, synergistic interventions that could significantly reduce malaria burden. IPT is crucial for pregnant women, but larviciding’s limited effects necessitate more effective strategies.
This demonstrates the relative priority for non-pyrethroid IRS and combined interventions such as ITNs plus IRS and emphasizes strengthening targeted approaches such as IPT for populations at high risk.
Conclusion
This systematic review highlighted the significant impact of integrated malaria prevention strategies in resource-limited settings, particularly in sub-Saharan Africa. Key interventions such as ITNs, IRS, and larviciding have been shown to significantly reduce malaria prevalence and incidence across various countries. The combined use of ITNs and IRS was demonstrated to have yielded remarkable reductions in malaria cases. Additionally, innovative approaches such as the use of natural repellents, community-based adaptive strategies, and improved data management systems further enhance the effectiveness of malaria control efforts. Despite challenges in implementation and sustainability, continuous and coordinated efforts remain essential for long-term success.
This review emphasises the need for local adaptation of malaria interventions, highlighting the importance of community engagement, operational capacity, and environmental factors, and the need for innovative strategies.
What is already known about the topic
- Integrated malaria prevention measures, like the combination of ITNs and IRS, are demonstrably more effective than isolated interventions, especially in sub-Saharan Africa.
- Community involvement and ongoing initiatives are vital for the efficacy and longevity of these treatments, as disengagement may result in a recurrence of malaria.
- Additionally, context-specific techniques and proficient data management are imperative for tackling local issues and tracking advancements. Insecticide resistance is a considerable challenge, requiring the formulation of alternative or supplementary strategies to sustain the efficacy of malaria control initiatives.
What this study adds
- This study provided substantial quantitative evidence for the synergistic application of ITNs and IRS, while underscoring the efficacy of innovative natural products such as neem seed oil and Dioscorea sansibarensis as viable alternatives or supplements to conventional interventions.
- It underscored the significance of adaptable techniques such as regular and early replacement of ITNs, increased ITN Coverage, education on proper ITN use and care, targeting temperature-sensitive parameters.
- Community acceptability, and enhanced data systems in improving malaria control, along with explicit dose-response relationships that validate the efficacy of interventions such as neem seed oil and integrated ITN-IRS methods.
- The study emphasized the necessity for context-specific tactics, ongoing monitoring to tackle resistance challenges, and the incorporation of contemporary technology, including social media campaigns, to enhance public engagement and knowledge in malaria control initiatives.
Funding
The authors did not receive any specific funding for this work.
Protocol Registration
The protocol for this review was registered in Prospero with registration number CRD420251032209 to provide methodological rigour and transparency.
Availability of data, code and other materials
Data and other materials used for this review are available upon request.
Authors´ contributions
Emmanuel George Bachan, Juliet Abu, Martin Nyaaba Adokiya and Charles Tobin-West conceived, designed and coordinated the systematic review data collection, Emmanuel George Bachan, Martin Nyaaba Adokiya and Charles Tobin-West screening, extraction and synthesis of literature and Emmanuel George Bachan, Juliet Abu, Martin Nyaaba Adokiya, Charles Tobin-West reviewed the manuscript. All the authors have read and approved the final manuscript.
Table 1: Quality ratings for study type and key outcomes using GRADE
| Study Design | Number of Studies | Initial Quality | Upgrades/Downgrades | Final Quality | Reference |
|---|---|---|---|---|---|
| Cross-sectional study | 3 | Low | Downgraded for risk of bias (due to observational nature), imprecision (small sample sizes) | Low | [20], [27], [15] |
| Laboratory-based experimental study | 1 | High | No downgrade (precise, well-conducted experimental study) | High | [17] |
| Mathematical model study | 1 | Moderate | Downgraded for indirectness (models may not fully reflect real-world complexity) | Low | [23] |
| Mixed-method study | 1 | Low | Upgraded for consistency and directness (results align with other intervention studies) | Moderate | [19] |
| Qualitative study | 1 | Low | Downgraded for imprecision and indirectness (limited generalizability, small sample) | Low | [14] |
| Before-and-after study | 1 | Moderate | Downgraded for bias and potential confounding (no control group) | Low | [22] |
| Retrospective cohort study | 1 | Moderate | Downgraded for bias (observational nature, potential confounding) | Low | [21] |
| Retrospective observational study | 2 | Moderate | Downgraded for imprecision (small sample sizes, retrospective nature introduces bias) | Low | [24], [13] |
| Retrospective study | 2 | Moderate | Downgraded for risk of bias (potential confounding, lack of prospective data) | Low | [18], [26] |
| Theoretical modeling study | 1 | Moderate | Downgraded for indirectness and imprecision (model assumptions, small case study applications) | Low | [25] |
| Randomized controlled trial (RCT) | 1 | High | No downgrade (randomization and blinding ensured high methodological rigor) | High | [16] |
Table 2: Pooled Effect Size and Heterogeneity Statistics
| Parameter | Estimate | 95% Confidence Interval | p-value |
|---|---|---|---|
| Effect Size (dˉ) | 1.27 | [0.58, 1.96] | < .001 |
| Heterogeneity Statistics | |||
| – Q (Residual Heterogeneity) | 105.97 | df = 8 | < .001 |
| – τ² (Between-Study Variance) | 1.06 | ||
| – τ (Standard Deviation) | 1.03 | ||
| – I² (%) | 98.08 | ||
| – H² | 52.17 | ||


References
- WHO. World Malaria Report 2024 [Internet]. Geneva (Switzerland): World Health Organization; 2024 Dec 11 [cited 2025 Sep 15]; 316p. Available from: https://www.who.int/publications/i/item/9789240104440 Download PDF to view full text
- Apeagyei AE, Patel NK, Cogswell I, O’Rourke K, Tsakalos G, Dieleman J. Examining geographical inequalities for malaria outcomes and spending on malaria in 40 malaria-endemic countries, 2010–2020. Malar J [Internet]. 2024 Jul 10 [cited 2025 Sep 15];23(1):206. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-024-05028-4 https://doi.org/10.1186/s12936-024-05028-4
- Nalinya S, Musoke D, Deane K. Malaria prevention interventions beyond long-lasting insecticidal nets and indoor residual spraying in low- and middle-income countries: a scoping review. Malar J [Internet]. 2022 Feb 2 [cited 2025 Sep 15];21(1):31. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-022-04052-6 https://doi.org/10.1186/s12936-022-04052-6
- CDC. Malaria’s Impact Worldwide [Internet]. Atlanta (GA): CDC; 2024 Apr 1 [cited 2024 Oct 22]. [about 7 screens]. Available from: https://www.cdc.gov/malaria/php/impact/index.html
- Pattanshetty S, Dsouza VS, Shekharappa A, Yagantigari M, Raj R, Inamdar A, Alsamara I, Rajvanshi H, Brand H. A scoping review on malaria prevention and control intervention in fragile and conflict-affected states (Fcas): a need for renewed focus to enhance international cooperation. J Epidemiol Glob Health [Internet]. 2024 Jan 15 [cited 2025 Sep 15];14(1):4–12. Available from: https://link.springer.com/10.1007/s44197-023-00180-7 https://doi.org/10.1007/s44197-023-00180-7 Subscription or purchase required to view full text
- Pryce J, Medley N, Choi L. Indoor residual spraying for preventing malaria in communities using insecticide-treated nets. Cochrane Infectious Diseases Group, editor. Cochrane Database of Systematic Reviews [Internet]. 2022 Jan 17 [cited 2025 Sep 15];2022(1): CD012688 Available from: http://doi.wiley.com/10.1002/14651858.CD012688.pub3 https://doi.org/10.1002/14651858.CD012688.pub3
- Awasthi KR, Jancey J, Clements ACA, Rai R, Leavy JE. Community engagement approaches for malaria prevention, control and elimination: a scoping review. BMJ Open [Internet]. 2024 Feb [cited 2025 Sep 15];14(2):e081982. Available from: https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2023-081982 https://doi.org/10.1136/bmjopen-2023-081982
- Awine T, Silal SP. Assessing the effectiveness of malaria interventions at the regional level in Ghana using a mathematical modelling application. Nacher M, editor. PLOS Glob Public Health [Internet]. 2022 Dec 21 [cited 2025 Sep 15];2(12):e0000474. Available from: https://dx.plos.org/10.1371/journal.pgph.0000474 https://doi.org/10.1371/journal.pgph.0000474
- Musoke D, Atusingwize E, Namata C, Ndejjo R, Wanyenze RK, Kamya MR. Integrated malaria prevention in low- and middle-income countries: a systematic review. Malar J [Internet]. 2023 Mar 6 [cited 2025 Sep 15];22(1):79. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-023-04500-x https://doi.org/10.1186/s12936-023-04500-x
- Ngwafor R, Pokharel S, Aguas R, White L, Shretta R. Models for malaria control optimization—a systematic review. Malar J [Internet]. 2024 Oct 3 [cited 2025 Sep 15];23(1):295. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-024-05118-3 https://doi.org/10.1186/s12936-024-05118-3
- Kombate G, Djalogue L, Ngangue P, Soubeiga KAM, Grobbee DE, Van Der Sande M. Integrated malaria vector control strategies and their effectiveness in sub-Saharan Africa: a systematic review protocol for interventional studies. BMJ Open [Internet]. 2025 Feb [cited 2025 Sep 15];15(2):e091569. Available from: https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2024-091569 https://doi.org/10.1136/bmjopen-2024-091569
- Alhassan Y, Dwomoh D, Amuasi SA, Nonvignon J, Bonful H, Tetteh M, Agyabeng K, Kotey M, Yawson AE, Bosomprah S. Impact of insecticide-treated nets and indoor residual spraying on self-reported malaria prevalence among women of reproductive age in Ghana: implication for malaria control and elimination. Malar J [Internet]. 2022 Apr 12 [cited 2025 Sep 15];21(1):120. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-022-04136-3 https://doi.org/10.1186/s12936-022-04136-3
- Gogue C, Wagman J, Tynuv K, Saibu A, Yihdego Y, Malm K, Mohamed W, Akplu W, Tagoe T, Ofosu A, Williams I, Asiedu S, Richardson J, Fornadel C, Slutsker L, Robertson M. An observational analysis of the impact of indoor residual spraying in Northern, Upper East, and Upper West Regions of Ghana: 2014 through 2017. Malar J [Internet]. 2020 Jul 11 [cited 2025 Sep 15];19(1):242. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-020-03318-1 https://doi.org/10.1186/s12936-020-03318-1
- Ndong IC, Okyere D, Enos JY, Amambua-Ngwa A, Merle CSC, Nyarko A, Koram KA, Ahorlu CS. Challenges and perceptions of implementing mass testing, treatment and tracking in malaria control: a qualitative study in Pakro sub-district of Ghana. BMC Public Health [Internet]. 2019 Jun 6 [cited 2025 Sep 15];19(1):695. Available from: https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-019-7037-1 https://doi.org/10.1186/s12889-019-7037-1
- Adedeji OA, Adetola BE, Modinat SM, Oyeneye AI. Intermittent preventive treatment and long-lasting insecticide nets use among pregnant women attending traditional birth homes in ibadan, nigeria. Journal of Interventional Epidemiology and Public Health [Internet]. 2023 Sep 6 [cited 2025 Sep 16];6(12). Available from: https://www.afenet-journal.net/content/article/6/12/full/
- Adedeji OA, Adetola BE, Modinat SM, Oyeneye AI. Intermittent preventive treatment and long-lasting insecticide nets use among pregnant women attending traditional birth homes in Ibadan, Nigeria. Journal of Interventional Epidemiology and Public Health [Internet]. 2023 Sep 6 [cited 2025 Sep 16];6(3):12. Available from: https://www.afenet-journal.net/content/article/6/12/full/ https://www.doi.org/10.37432/jieph.2023.6.3.84
- Philbert A. Larvicidal potency of Dioscorea sansibarensis leaf extract against vector mosquitoes: Anopheles gambiae s.s. and Culex quinquefasciatus. Tanz J Sci [Internet]. 2021 May 27 [cited 2025 Sep 16];47(2):655–63. Available from: https://www.ajol.info/index.php/tjs/article/view/207366 https://doi.org/10.4314/tjs.v47i2.21 Download PDF to view full text
- Killeen GF, Govella NJ, Mlacha YP, Chaki PP. Suppression of malaria vector density and human infection prevalence associated with scale-up of mosquito-proofed housing in Dar es Salaam, Tanzania: re-analysis of an observational series of parasitological and entomological surveys. The Lancet Planetary Health [Internet]. 2019 Mar [cited 2025 Sep 16];3(3):e132–43. Available from: https://www.thelancet.com/journals/lanplh/article/PIIS2542-5196(19)30035-X/fulltext https://doi.org/10.1016/S2542-5196(19)30035-X
- Matindo AY, Kapalata SN, Katalambula LK, Meshi EB, Munisi DZ. Biolarviciding for malaria vector control: Acceptance and associated factors in southern Tanzania. Current Research in Parasitology & Vector-Borne Diseases [Internet]. 2021 Jun 23 [cited 2025 Sep 16];1:100038. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2667114X21000327 https://doi.org/10.1016/j.crpvbd.2021.100038
- Metelo-Matubi E, Zanga J, Binene G, Mvuama N, Ngamukie S, Nkey J, Schopp P, Bamba M, Irish S, Nguya-Kalemba-Maniania J, Fasine S, Nagahuedi J, Muyembe JJ, Mansiangi P. The effect of a mass distribution of insecticide-treated nets on insecticide resistance and entomological inoculation rates of Anopheles gambiae S.l. in Bandundu City, Democratic Republic of Congo. Pan Afr Med J [Internet]. 2021 Oct 25 [cited 2025 Sep 16];40:118. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8627145/ https://doi.org/10.11604/pamj.2021.40.118.27365
- Dirontsho M, Barbara Ntombi N, Oluwatoyin Dare K, Moseki Ronald M, Vincent P. Local knowledge of adaptive strategies against malaria endemicity in the Okavango Delta, Botswana. AJID [Internet]. 2022 May 12 [cited 2025 Sep 16];16(2):21–34. Available from: https://journals.athmsi.org/index.php/AJID/article/view/5825 https://doi.org/10.21010/Ajid.v16i2.3
- Mohammed MA, Hong T. Role of vector control in fighting against malaria: Evidence from Ethiopian health-related indicators. Journal of Infection and Public Health [Internet]. 2021 Mar 18 [cited 2025 Sep 16];14(4):527–32. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1876034120307486 https://doi.org/10.1016/j.jiph.2020.12.002
- Ochieng FO. SEIRS model for malaria transmission dynamics incorporating seasonality and awareness campaign. Infectious Disease Modelling [Internet]. 2024 Mar [cited 2025 Sep 16];9(1):84–102. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2468042723001069 https://doi.org/10.1016/j.idm.2023.11.010
- Wagman J, Gogue C, Tynuv K, Mihigo J, Bankineza E, Bah M, Diallo D, Saibu A, Richardson JH, Kone D, Fomba S, Bernson J, Steketee R, Slutsker L, Robertson M. An observational analysis of the impact of indoor residual spraying with non-pyrethroid insecticides on the incidence of malaria in Ségou Region, Mali: 2012–2015. Malar J [Internet]. 2018 Jan 10 [cited 2025 Sep 16];17(1):19. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-017-2168-2 https://doi.org/10.1186/s12936-017-2168-2
- Ngonghala CN. The impact of temperature and decay in insecticide-treated net efficacy on malaria prevalence and control. Mathematical Biosciences [Internet]. 2023 Jan [cited 2025 Sep 16];355:108936. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0025556422001250 https://doi.org/10.1016/j.mbs.2022.108936
- Rae JD, Nosten S, Kajeechiwa L, Wiladphaingern J, Parker DM, Landier J, Thu AM, Dah H, Be A, Cho WC, Paw K ’Nyaw, Paw ES, Shee PB, Poe C, Nu C, Nyaw B, Simpson JA, Devine A, Maude RJ, Moo KL, Min MC, Thwin MM, Tun SW, Nosten FH. Surveillance to achieve malaria elimination in Eastern Myanmar: a 7-year observational study. Malar J [Internet]. 2022 Jun 7 [cited 2025 Sep 16];21(1):175. Available from: https://malariajournal.biomedcentral.com/articles/10.1186/s12936-022-04175-w https://doi.org/10.1186/s12936-022-04175-w
- Alhassan RK, Nketiah-Amponsah E, Arhinful DK. A review of the national health insurance scheme in ghana: what are the sustainability threats and prospects? Coles JA, editor. PLoS ONE [Internet]. 2016 Nov 10 [cited 2025 Sep 16];11(11):e0165151. Available from: https://dx.plos.org/10.1371/journal.pone.0165151 https://doi.org/10.1371/journal.pone.0165151
