Research | Open Access | Volume 8 (4): Article 92 | Published: 14 Nov 2025
Evaluation of praziquantel efficacy in children after two decades of mass distribution in Bamako, Mali
Menu, Tables and Figures
On Pubmed
Navigate this article
Tables
| Variables | Frequency (n=99) | Percentage (%) |
|---|---|---|
| Sites | ||
| Taliko | 82 | 82.8 |
| Missabougou | 17 | 17.2 |
| Gender | ||
| Male | 52 | 52.5 |
| Female | 47 | 47.5 |
| Age | ||
| 6–10 | 18 | 18.2 |
| 11–14 | 81 | 81.8 |
| Taking traditional medicines | ||
| No | 61 | 61.6 |
| Yes | 38 | 38.4 |
| River frequentation | ||
| Yes | 77 | 77.8 |
| No | 22 | 22.2 |
| Habitat–River Distance | ||
| < 50 m | 94 | 94.9 |
| ≥ 50 m | 5 | 5.1 |
| Domestic water source | ||
| River | 1 | 1 |
| Well | 1 | 1 |
| Tap | 82 | 82.8 |
| Borehole | 15 | 15.2 |
| Parents profession | ||
| Shopkeeper | 10 | 10.1 |
| Civil servant | 5 | 5.1 |
| Gardener | 1 | 1 |
| Manual labor | 82 | 82.8 |
| Fisherman | 1 | 1 |
| S. haematobium | ||
| Yes | 77 | 77.8 |
| No | 22 | 22.2 |
| S. mansoni | ||
| Yes | 35 | 35.4 |
| No | 64 | 64.6 |
| Coinfected | ||
| Yes | 13 | 13.1 |
| No | 86 | 86.9 |
Table 1. Distribution of children included in the study according to sociodemographic, socioeconomic, and infection status (N = 99)
| Clinical signs | Pre-treatment n=77 (%) | p | Post-treatment n=15 (%) | p |
|---|---|---|---|---|
| Bladder pain | ||||
| No | 36 (46.8) | 0.65 | 8 (53.3) | >0.05 |
| Yes | 41 (53.2) | 7 (46.7) | ||
| Abdominal pain | ||||
| No | 32 (41.6) | 0.17 | 5 (33.3) | 0.30 |
| Yes | 45 (58.4) | 10 (66.7) | ||
| Pollakiuria | ||||
| No | 58 (75.3) | <0.001 | 10 (66.7) | 0.30 |
| Yes | 19 (24.7) | 5 (33.3) | ||
| Dysuria | ||||
| No | 51 (66.2) | 0.006 | 8 (53.3) | >0.05 |
| Yes | 26 (33.8) | 7 (46.7) |
Table 2. Distribution of Schistosoma haematobium–infected children included in the cohort according to clinical signs at both study sites (N = 77)
| Demographic variable | Number Examined (%) | Number cured (Cure Rate) | 95% CI | p | AEMC before treatment | AEMC after treatment | ERR (%) | |
|---|---|---|---|---|---|---|---|---|
| Sites | ||||||||
| Taliko | Low | 63 (91.3) | 50 (79.4) | [68.4–88.1] | <0.0001 | 5 | 7 | 83.8 |
| High | 6 (8.7) | 4 (66.7) | [22.3–95.7] | 72 | 0 | 89.5 | ||
| Total | 69 | 54 (78.3) | [68.4–88.1] | 87.0 | ||||
| Missabougou | Low | 8 | 8 (100.0) | [63.1–100] | 2 | 0 | 100 | |
| High | 0 | – | – | – | – | – | ||
| Total | 8 (100.0) | 8 (100.0) | [63.1–100] | 100 | ||||
| Gender | ||||||||
| Female | Low | 36 (100) | 30 (83.3) | [71.1–95.5] | 0.89 | 6 | 4 | 90.3 |
| High | 0 | – | – | – | – | – | ||
| Total | 36 | 30 (83.3) | [71.1–95.5] | 90.3 | ||||
| Male | Low | 35 (85.4) | 28 (80) | [63.9–91.6] | 4 | 9 | 74.4 | |
| High | 6 (14.6) | 4 (66.7) | [22.3–95.7] | 72 | – | 89.5 | ||
| Total | 41 | 32 (78.0) | [65.5–90.5] | 86.0 | ||||
| Age (years) | ||||||||
| 6–10 | Low | 10 (90.0) | 9 (90) | [55.5–99.7] | <0.0001 | 7 | 2 | 96.4 |
| High | 1 (10.0) | 1 (100) | [2.5–100] | 52 | – | 100 | ||
| Total | 11 | 10 (90.9) | [74.1–100] | 98.1 | ||||
| 11–14 | Low | 61 (92.4) | 49 (80.3) | [68.8–88.9] | 5 | 7 | 82.3 | |
| High | 5 (7.6) | 3 (60) | [14.7–94.7] | 75 | – | 88.1 | ||
| Total | 66 | 52 (78.8) | [69.0–88.6] | 85.5 | ||||
| Total | 77 | 62 (80.5) | [71.2–89.8] | 87.3 | ||||
*Arithmetic egg mean counts (AEMC)
Table 3. Cure rate (CR) and egg reduction rate (ERR) observed for S. haematobium infection in children in relation to sociodemographic factors and infection intensity in Taliko and Missabougou sites, N=77
| Demographic variable | Subcategory | OR | 95% CI | p-value |
|---|---|---|---|---|
| Site | Missabougou | — | — | — |
| Taliko | 1.4 | 0.00–5.80 | >0.9 | |
| Gender | Female | — | — | — |
| Male | 1.76 | 0.55–5.95 | 0.3 | |
| Age (years) | 6–10 | 0.35 | 0.02–2.15 | 0.3 |
| 11–14 | — | — | — |
OR = Odds Ratio, CI = Confidence Interval
Table 4. Multivariate analysis of sociodemographic variables as a function of S. haematobium infection, N = 77
| Demographic variable | Subcategory | Number Examined (%) | Number cured (CR) | 95% CI | p | AEMC before treatment | AEMC after treatment | ERR (%) |
|---|---|---|---|---|---|---|---|---|
| Site | ||||||||
| Taliko | Low | 6 (23.1) | 5 (83.3) | [35.9 – 99.6] | 0,002 | 72 | 84 | 39 |
| Moderate | 4 (15.4) | 3 (75) | [19.4 – 99.4] | 0,002 | 354 | 264 | 91.5 | |
| High | 16 (61.5) | 15 (93.8) | [69.8 – 99.8] | 0,002 | 1512 | – | 99.8 | |
| Total | 26 | 25 (96.2) | [80.4 – 99.9] | 0,002 | – | – | 98.3 | |
| Missabougou | Low | 8 (88.9) | 6 (75) | [34.9 – 96.8] | 0,002 | 51 | 60 | 70.6 |
| Moderate | 0 | – | [2.5 – 100] | 0,002 | – | – | – | |
| High | 1 (11.1) | 1 (100) | [40.0 – 97.2] | 0,002 | 1224 | – | 100 | |
| Total | 9 | 7 (77.8) | [35.9 – 99.6] | 0,002 | – | – | 92.6 | |
| Gender | ||||||||
| Female | Low | 3 (17.6) | 3 (100) | [29.2 – 100] | 0,86 | 56 | 84 | 100 |
| Moderate | 3 (17.6) | 2 (66.7) | [9.4 – 99.2] | 0,86 | 344 | – | 88.4 | |
| High | 11 (64.8) | 10 (90.9) | [58.7 – 99.8] | 0,86 | 1210 | – | 99.6 | |
| Total | 17 | 15 (88.2) | [63.6 – 98.5] | 0,86 | – | – | 98.8 | |
| Male | Low | 11 (61.2) | 8 (72.7) | [39.0 – 93.9] | 0,86 | 61 | 60 | 42.9 |
| Moderate | 1 (5.5) | 1 (100) | [2.5 – 100] | 0,86 | 384 | 264 | 100 | |
| High | 6 (33.3) | 6 (100) | [54.1 – 100] | 0,86 | 2016 | – | – | |
| Total | 18 | 17 (94.4) | [72.7 – 99.9] | 0,86 | – | – | 97.1 | |
| Age (years) | ||||||||
| 6-10 | Low | 4 (57.1) | 4 (100) | [39.8 – 100] | 0,0005 | 66 | 48 | 100 |
| Moderate | 0 | – | [9.4 – 99.2] | 0,0005 | – | – | – | |
| High | 3 (42.9) | 2 (66.7) | [42.1 – 99.6] | 0,0005 | 1344 | – | 98.8 | |
| Total | 7 | 6 (85.7) | [34.8 – 93.3] | 0,0005 | – | – | 98.9 | |
| 11-14 | Low | 10 (35.7) | 7 (70) | [19.4 – 99.4] | 0,0005 | 58 | 80 | 33.3 |
| Moderate | 4 (14.3) | 3 (75) | [76.8 – 100] | 0,0005 | 354 | 264 | 91.5 | |
| High | 14 (50.0) | 14 (100) | [67.3 – 96.0] | 0,0005 | 1527 | – | 100 | |
| Total | 28 | 24 (85.7) | [76.9 – 98.2] | 0,0005 | – | – | 97.8 | |
| Total | 35 | 30 (85.7) | [39.8 – 100] | – | – | – | 98 | |
*Arithmetic egg mean counts (AEMC)
Table 5. Cure Rate (CR) and Egg Reduction Rate (ERR) Observed in Schistosoma mansoni-Infected Children According to Sociodemographic Variables and Infection Intensity, N=35
| Demographic variable | Subcategory | OR | 95% CI | p-value |
|---|---|---|---|---|
| Site | Missabougou | — | — | — |
| Taliko | 0.48 | 0.06, 4.71 | 0.5 | |
| Gender | Female | — | — | — |
| Male | 1.30 | 0.15, 14.9 | 0.8 | |
| Age (years) | 6-10 | 0.95 | 0.04, 13.5 | >0.9 |
| 11-14 | — | — | — |
OR = Odds Ratio, CI = Confidence Interval
Table 6. Multivariate analysis of sociodemographic variables as a function of S. mansoni infection, N=35
Figures
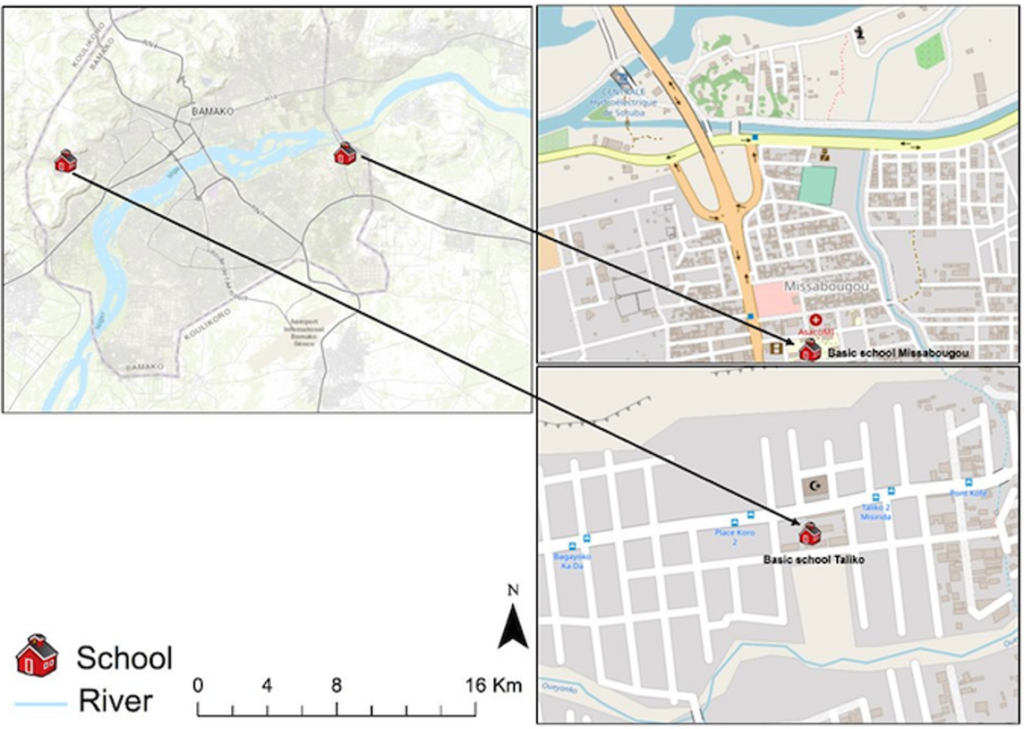
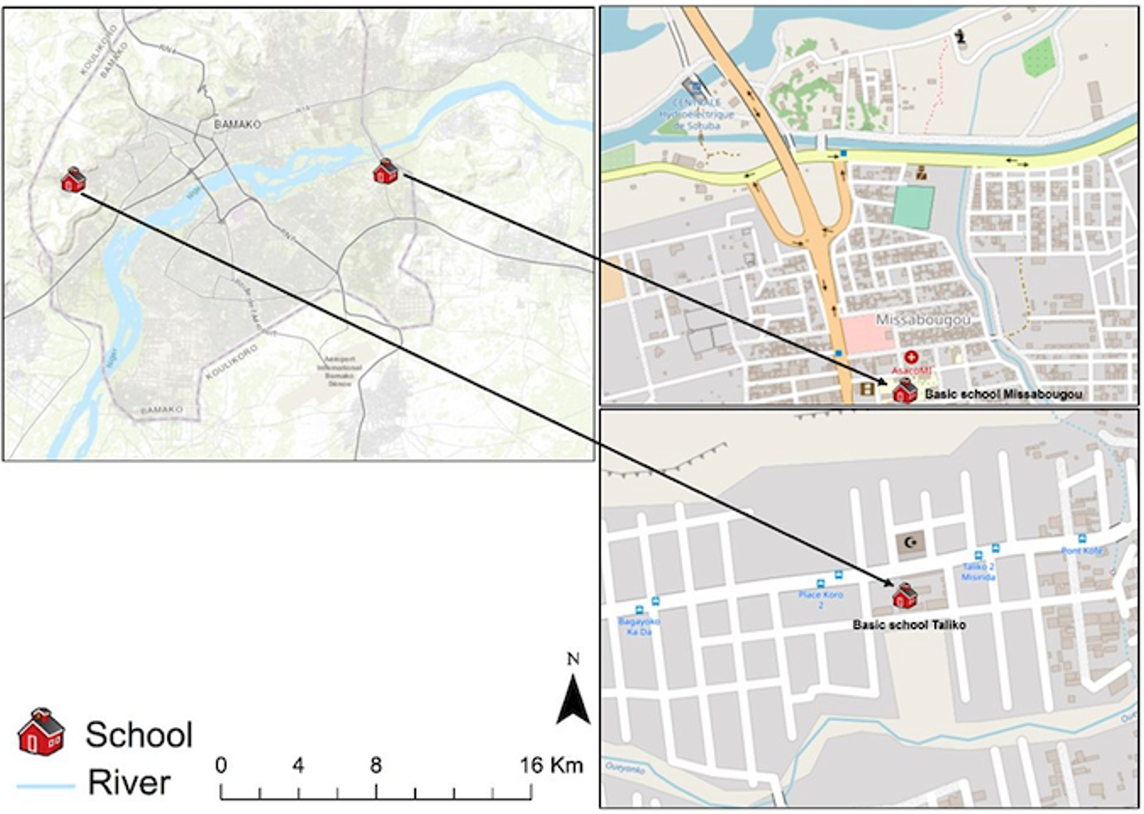

Figure 2: Distribution of mumps attack rates by district, Western North Region, August 2022


Keywords
- Schistosomiasis
- Efficacy
- Praziquantel
- Cure rate
- Egg reduction rate
- Bamako
- Mali
Privat Agniwo1,2,&, Laurent Dembélé1, Abdoulaye Koné1, Bakary Sidibé1, Ahmed Konaté1, Adam Garango1, Rabiatou Diarra1,Assitan Diakité1, Ahristode Akplogan1, Hassim Guindo1, Ornela Dossa1, Oumar Coulibaly3, Boubacar Traoré1, Mahamadou Ali Thera1, Abdoulaye Djimdé1, Abdoulaye Dabo1, Safiatou Niare Doumbo1,&
1Department of Epidemiology of Parasitic Diseases, Faculty of Pharmacy, University of Sciences, Techniques and Technologies of Bamako, Bamako, Mali, 2Centre de Recherche pour la lutte contre les Maladies Infectieuses Tropicales (CReMIT/TIDRC), Université d’Abomey-Calavi, Calavi, Benin, 3Institut National De Recherche En Santé Publique, Bamako, Mali
&Corresponding author: Privat Agniwo, Department of Epidemiology of Parasitic Diseases, Faculty of Pharmacy, University of Sciences, Techniques and Technologies of Bamako, Bamako, Mali,
Email: privatagniwo@yahoo.com, ORCID: https://orcid.org/0000-0002-6913-490X,
Safiatou Niare Doumbo, Department of Epidemiology of Parasitic Diseases, Faculty of Pharmacy, University of Sciences, Techniques and Technologies of Bamako, Bamako, Mali,
Email: sdoumbo@icermali.org
Received: 04 Jul 2025, Accepted: 13 Nov 2025, Published: 14 Nov 2025
Domain: Neglected Tropical Diseases
Keywords: Schistosomiasis, Efficacy, Praziquantel, Cure rate, Egg reduction rate, Bamako, Mali
©Privat Agniwo et al. Journal of Interventional Epidemiology and Public Health (ISSN: 2664-2824). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Privat Agniwo et al., Evaluation of Praziquantel efficacy in children after two decades of Mass distribution in Bamako, Mali. Journal of Interventional Epidemiology and Public Health. 2025;8(4):92. https://doi.org/10.37432/jieph-d-25-00154
Abstract
Introduction: Schistosomiasis, a neglected tropical disease, remains endemic in Mali despite ongoing preventive chemotherapy with praziquantel (PZQ). This study aimed to assess the therapeutic efficacy of PZQ against Schistosoma haematobium and S. mansoni infections in school-aged children residing in urban and peri-urban areas of the Bamako district.
Methods: A prospective cohort study was conducted between April and May 2023 among children aged 6 to 14 years who tested positive for schistosomiasis, recruited from an epidemiological survey involving 736 children. Data on sociodemographic, socioeconomic, clinical, and parasitological variables were collected at baseline (Day 0) and 14 days after PZQ treatment. Treatment was administered on Day 0 according to WHO recommendations (40 mg/kg). Parasitological diagnosis was performed using the urine filtration technique for S. haematobium and the Kato-Katz method for S. mansoni at both Day 0 and Day 14. Treatment efficacy was assessed using the cure rate (CR) and the egg reduction rate (ERR).
Results: A total of 99 children infected with schistosomiasis were followed up, including 77 children positive for S. haematobium, 35 for S. mansoni, and 13 who were co-infected. Among these 99 children, 82.8% resided in Taliko and 17.2% in Missabougou. Most Taliko children lived within 500 meters of the Woyowayanko River and had frequent contact with it. The overall CR for S. haematobium was 80.5%, with an ERR of 87.3%. In Taliko, the highest ERR (79.4%) was observed among children with low parasite burden, while in Missabougou, all children with low infection intensity were egg-negative two weeks after treatment.
Conclusion: The 14-day follow-up was chosen in accordance with WHO recommendations for assessing short-term efficacy and egg clearance after praziquantel (PZQ) treatment. Although the results suggest a reduction in infection levels among treated children, the limited follow-up period does not allow firm conclusions on long-term efficacy or reinfection dynamics. The relatively lower cure and egg reduction rates observed in some subgroups may indicate the need for extended follow-up and sustained monitoring, as well as targeted interventions such as health education and community engagement—to enhance schistosomiasis control and elimination outcomes.
Introduction
Schistosomiasis is one of the neglected tropical diseases (NTDs) among the five targeted by preventive chemotherapy (NTD-PC) in Mali [1]. It is a parasitic disease endemic in tropical and subtropical regions [2]. In 2021, chemoprevention against schistosomiasis was required for 51 countries for a total of 251.4 million people, including 136 million school-age children [3] with 90% of cases in sub-Saharan Africa [4]. Schistosome species have a two-host life cycle, with an asexual stage in a freshwater snail host and a sexual stage in the definitive mammalian host. Parasite eggs are excreted in the urine (Schistosoma haematobium) or in the feces for all other schistosome species found in humans (Schistosoma mansoni, S. guineensis, S. intercalatuùm, S. mekongi and S. japonicum). The worldwide distribution of schistosomes shows that of the six species infecting humans, S. haematobium and S. mansoni (responsible for urogenital and intestinal schistosomiasis respectively) are the most endemic in Africa [5,6].
In Mali, Schistosomiasis caused by Schistosoma haematobium and S. mansoni is endemic, with an overall prevalence of 38.3% for the former species versus 6.7% for the latter [7]. The villages most affected are those along the Niger and Senegal rivers, those on the Dogon Plateau and in the Office du Niger, and those around large ponds in the Diéma cercle [8,9]. While S. haematobium is the most widespread species, with a prevalence of up to 78%, S. mansoni is locally distributed, with prevalences in the Office du Niger exceeding 50% in the general population [10].
In all national schistosomiasis control programs, a single drug, praziquantel (PZQ), is currently used for the control and treatment of all forms of schistosomiasis. According to World Health Organization (WHO) recommendations, a single 40 mg/kg dose of PZQ is required as preventive chemotherapy both in whole communities (as mass treatment) or in school-age children in areas where the prevalence of infection is sufficiently high; however, the drug could additionally be used to treat infected individuals in areas of low transmission [11]. According to the World Health Organization (WHO) guidelines on the control and elimination of human schistosomiasis, the frequency of mass drug administration (MDA) is determined based on the prevalence of Schistosoma spp. infection among school-aged children (SAC): High prevalence (≥50%): Biannual MDA for all SAC; Moderate prevalence (10–49.9%): MDA annually for all SAC [12]. But in addition to school-age children considered the main target of interventions, younger children (of preschool age) are now recognized as a vulnerable population. However, data on this age group are relatively limited [12].
For over a decade, since 2006, PZQ has been used systematically as a preventive chemotherapy for the control of schistosomiasis worldwide. But it should be noted that this strategy is also used in the case of other helminthiases such as lymphatic filariasis, onchocerciasis, geohelminthiases, hookworm, ascariasis, trichocephalosis and the bacterial infection causing trachoma [3]. The revised WHO roadmap for neglected tropical diseases targets the elimination of schistosomiasis as a public health issue and the interruption of its transmission in endemic regions by 2030, supporting progress toward Sustainable Development Goal 3 [3].
Mali was among the first countries in sub-Saharan Africa to launch a schistosomiasis control initiative, beginning in 1972. The initial program targeted two major endemic areas: the Bandiagara area on the Plateau Dogon and the irrigated rice-growing region of the Office du Niger, known for its small dams. In 1982, the country established the National Schistosomiasis and Geohelminthiasis Control Program (PNLSH) to strengthen the fight against the disease. The program’s main objective was to reduce morbidity through a strategy centered on mass drug administration (MDA) using a single dose of praziquantel (PZQ) at 40 mg/kg—an approach widely adopted in other endemic countries as well [13–15].
Since then, annual or biannual treatment campaigns have been conducted in endemic areas, based on the prevalence thresholds currently recommended by the WHO. In 2005, the PNLSH was integrated into the broader Programme de Lutte contre les Maladies Tropicales Négligées (MTN). Mass distribution of praziquantel (PZQ) has continued under this integrated framework since 2007, with financial support from USAID/RTI/HKI, WHO, and Sightsavers. Despite these sustained efforts, unexpectedly high prevalence peaks have been observed in some endemic areas even after PZQ treatment, raising concerns about the effectiveness of current control strategies [16–18]. This study aimed to assess the efficacy of praziquantel against Schistosoma haematobium and S. mansoni infections among school-aged children in the Bamako district.
Methods
Study setting
The Bamako district is composed of six communes and over fifty neighborhoods. As of October 2019, its population was estimated at approximately 3,007,122 inhabitants. Bamako, the capital city of Mali, spans an area of 1,420 km² and is intersected by the Niger River and its tributaries. It is situated within the northern Sudanese climatic zone, which is characterized by two distinct seasons. The rainy season occurs from June to November, often accompanied by intense thunderstorms and heavy runoff events at its onset and conclusion. The mean annual precipitation is approximately 1,400 mm. The dry season extends from December to May, with consistently elevated temperatures, averaging 33°C annually [19]. The Bamako district is equipped with four university hospital centers (CHUs): Gabriel Touré, Point G, CNOS, and Hôpital du Mali. Each of the six communes within the district is served by a referral health center, in addition to a network of community health centers and numerous private medical facilities. Regarding the educational infrastructure, the district encompasses two basic education academies, located on either side of the Niger River. Each commune houses one or two Centres d’Animation Pédagogique (CAP), depending on the density of schools within its area. During the present study, both public and private schools were selected for sampling across the different identified ecological zones.
The study was conducted in two distinct areas of the Bamako district: Missabougou (2.637°N, 7.921°W), located in Commune VI (CVI), which is undergoing significant social and environmental changes; and Taliko (12.626°N, 8.059°W), a peri-urban zone situated in Commune IV (CIV) of Bamako (Figure 1).
In the peri-urban area of Taliko, the Woyowayanko tributary of the Niger River was initially engineered to serve as a rainwater drainage system. However, progressive residential encroachment along and adjacent to the watercourse has occurred, resulting in its concurrent use for the discharge of domestic wastewater. The Taliko educational facility is located in close proximity to this water body.
Missabougou has experienced significant environmental changes, including the rehabilitation of the canal bordering the neighborhood and the construction of bridges to enhance pedestrian connectivity across the waterway. The area benefits from a reliable potable water supply. Strategically situated near Bamako’s third bridge, Missabougou hosts several key infrastructures, including a modern hospital, and is traversed by a major four-lane arterial road providing direct access to National Route 6 (RN°6) (Figure 1).
Study design, population and duration
The findings presented herein are derived from a larger initial epidemiological investigation of schistosomiasis conducted in the Taliko and Missabougou districts [20]. However, due to its specific focus, the current study concentrated exclusively on the follow-up of individuals who tested positive during the initial screening and subsequently received treatment. The base study population comprised 736 schoolchildren aged 6 to 14 years enrolled in basic schools in Taliko and Missabougou, specifically those in grades 4, 5, and 6 [20]. This cohort was selected based on the documented peak prevalence and intensity of schistosomiasis [20] and geohelminthiasis within this age group, as well as because infection levels in these pupils provide a relevant measure of the impact of repeated mass drug administration following the implementation of control programs. Exclusion criteria included: (i) inability to provide biological samples during follow-up; (ii) presence of severe concomitant medical conditions; (iii) occurrence of diarrhea at the time of initial sampling; (iv) suspected pregnancy; (v) history of severe adverse reactions to praziquantel; and (vi) unavailability for follow-up on Day 14. The study employed a prospective cohort design with two cross-sectional surveys conducted 14 days apart, carried out in April and May 2023.
Sampling
Sociodemographic and economic data collection
Socio-demographic characteristics, including participants’ age, sex, and parental occupation, along with detailed information on human-water contact patterns, were obtained via a structured questionnaire. Data encompassed habitat-River Distance (<50 m and≥ 50 m), River frequentation, the types of water sources utilized (e.g., well, river, Tap, rainwater, Borehole), Taking traditional medicines, and parents’ profession. These parameters were selected to quantify direct exposure to contaminated aquatic environments, which are recognized as the primary factors in schistosomiasis transmission, and to enable the analysis of the relationships between behavior, environmental context, and infection risk.
Clinical data collection
The clinical examination was limited to symptoms indicative of urogenital schistosomiasis, including bladder and abdominal pain, pollakiuria, and dysuria. A single general practitioner, blinded to the infection status of the participants, performed all assessments.
Procedures for parasitological data collection
Participants involved in both the initial and follow-up phases of the study were provided with two labeled 125 ml containers and instructed to submit a fresh morning stool sample as well as a urine sample collected between 10 a.m. and 2 p.m. Both urine and stool specimens were processed following standardized parasitological protocols as previously described [20]. The intensity of Schistosoma haematobium infection was measured as the number of eggs per 10 ml of urine and classified into three categories based on WHO guidelines: (i) no eggs detected; (ii) light infection (1–49 eggs per 10 ml of urine); and (iii) heavy infection (≥50 eggs per 10 ml of urine). Schistosoma mansoni infection intensity was expressed as eggs per gram (EPG) of stool and categorized into four WHO-defined classes: (i) no eggs; (ii) light infection (1–99 EPG); (iii) moderate infection (100–399 EPG); and (iv) heavy infection (≥400 EPG) [20]. Quality control procedures involved re-examination of 10% of randomly selected filters and slides by an independent, experienced biologist.
Participant handling procedure
All children found positive for S. haematobium and/or S. mansoni [20] received praziquantel (PZQ) treatment at a dose of 40 mg/kg body weight, administered under direct supervision following the recommendations of Mali’s National Program for the Control of Schistosomiasis and Soil-Transmitted Helminths (PNLSH). Before treatment, each child was given doughnuts to reduce potential gastrointestinal side effects. Dosage was calculated based on individual body weight, measured with a calibrated scale, to ensure accurate dosing.
Post-treatment, children were observed for approximately four hours at school while engaging in normal activities. Any adverse effects were promptly reported to the school administration. Participants who vomited following PZQ administration were excluded from the analysis. Fourteen days after treatment, stool and urine samples were collected again. Children who continued to excrete schistosome eggs were retreated with the same regimen. During follow-up, participants infected with other helminths were treated with a single oral dose of either 400 mg albendazole or 500 mg mebendazole, administered under supervision after providing doughnuts to each child.
Follow-up of treated children
The efficacy assessment of praziquantel targeted children who tested positive for Schistosoma haematobium and/or S. mansoni during the initial epidemiological survey. All infected children received a single oral dose in Day 0 of praziquantel (40 mg/kg), in accordance with WHO recommendations for preventive chemotherapy in endemic areas. Fourteen days post-treatment, the children were re-evaluated to assess therapeutic outcomes, following WHO (2013) guidelines for short-term efficacy studies [21]. Stool and/or urine samples were collected during this second survey round to determine egg counts and calculate the cure rate (CR) and egg reduction rate (ERR).
The 14-day follow-up period was specifically chosen to minimize the confounding effect of reinfection, which can occur rapidly in endemic settings. This interval aligns with WHO recommendations, which suggest that post-treatment assessments be conducted sufficiently early (2–3 weeks) to distinguish between true treatment failure and new infections, while allowing adequate time for praziquantel to act on adult worms [21]. During follow-up, the same trained laboratory personnel conducted standardized parasitological examinations for urine and stool samples, ensuring consistency and comparability of results.
Praziquantel (PZQ) treatment efficacy
The efficacy of praziquantel was evaluated using two metrics: the cure rate (CR), defined as the proportion of children testing negative after treatment relative to the number initially positive, expressed as a percentage; and the egg reduction rate (ERR), calculated following the formula recommended by the WHO (2013).
$$
Cure Rate (CR) =
\left(
\frac{
\text{No. of children no longer excreting eggs in urine or stools after treatment}
}{
\text{No. of children eliminating eggs in urine or stool before treatment}
}
\right)
\times 100
$$
$$
Egg reduction rate (ERR) = 1 –
\left(
\frac{
\text{Arithmetic mean number of eggs at time of monitoring}
}{
\text{Arithmetic mean number of eggs at start}
}
\right)
$$
Data analysis
Data were systematically collected using case report forms, digitized in Microsoft Excel, and subjected to statistical analysis via IBM SPSS Statistics (version 23). Age was stratified into two categories: 6–10 years and 11–14 years. Multivariate logistic regression models were employed to investigate associations between parasitic infection status and demographic factors. The binary variable (positive/negative) was included in the multivariate logistic regression model for each Schistosoma species to assess associations with demographic factors. Proportional differences were assessed using Pearson’s Chi-square test or Fisher’s exact test when applicable. Due to the small sample size, 95% confidence intervals for the cure rates (CR) were calculated using the exact binomial method (Clopper-Pearson). Quantitative variables (egg counts) were presented as arithmetic means and standard deviations (AEMC). A univariate analysis was first performed to assess the associations between infection status and sociodemographic variables (study site, sex, age, water contact, distance between home and river, and parents’ occupation). The multivariate analysis using a logistic regression model was performed to estimate adjusted odds ratios (ORs) and their 95% confidence intervals (95% CI). A threshold of p < 0.05 was applied to determine statistical significance.
Ethical considerations
The study protocol was reviewed and approved by the Institutional Ethics Committee (IEC) of the Faculty of Medicine and Odontology and the Faculty of Pharmacy at the University of Sciences, Techniques, and Technologies of Bamako (Approval No. 2023/69/CE/USSTB). Before the initiation of the study, oral consent was obtained from teachers as well as from the parents or guardians of the participating children. However, written consent was also obtained from the school principals, and assent was obtained from children aged over 12 years. All participants who tested positive for one or both Schistosoma species received praziquantel treatment at 40 mg/kg body weight administered postprandially, in accordance with the guidelines of Mali’s National Program for the Control of Schistosomiasis and Soil-Transmitted Helminths (PNLSH). Participants who remained positive following initial treatment were retreated accordingly. Concurrent helminth infections were managed with albendazole. To ensure confidentiality and data protection, each participant was assigned a unique identification number and securely stored data with restricted access limited to authorized investigators.
Results
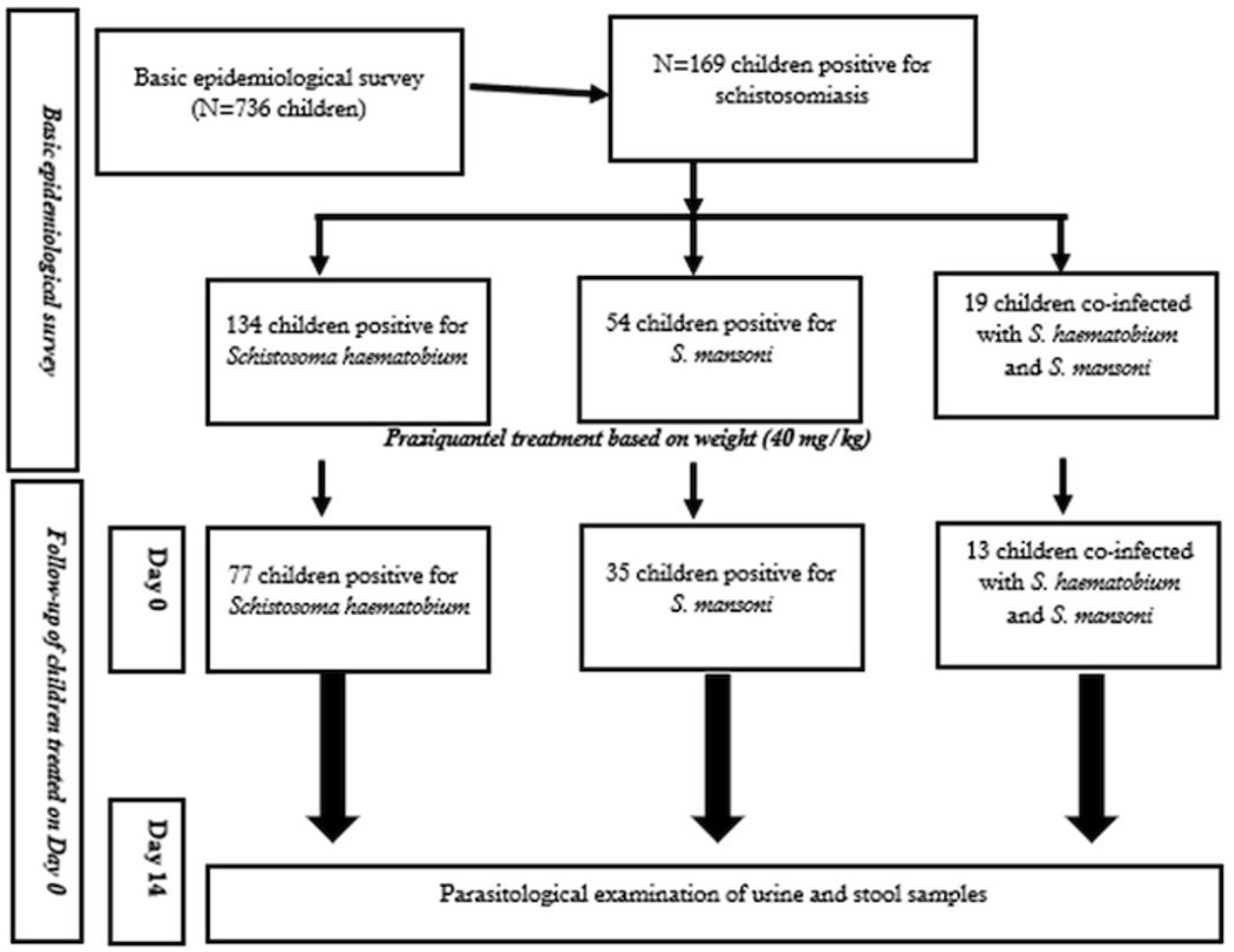
Of the 99 children successfully followed up and resampled during the second survey (Day 14), 77 were initially positive for S. haematobium, 35 for S. mansoni, and 13 were coinfected with both species at baseline (Figure 2). The majority of the followed-up participants resided in Taliko (82.8%, 82/99), while 17.2% (17/99) were from Missabougou. Socioeconomic data were collected for all 99 children at follow-up, whereas clinical assessments were performed only for those infected with S. haematobium (n = 77).
Socio-demographic and socio-economic characteristics of participants at both sites
The majority of children included in this study originated from Taliko, representing 82.8% (82/99) of the cohort. The sample was predominantly composed of older children aged 11 to 14 years and males (Table 1). Regarding socioeconomic factors, 38.4% of the participants reported prior use of traditional medicines for schistosomiasis treatment. A substantial proportion (77.8%) frequently accessed the Woyowayanko River, with 94.9% residing within less than 50 meters of this water source. Additionally, 82.8% of children reported using tap water for household purposes. The majority of parents were engaged in manual labor (82.8%), followed by shopkeepers (10.1%) (Table 1).
Clinical signs observed before and after treatment
Clinical data collected at baseline indicated that most children infected with urogenital schistosomiasis (S. haematobium) did not exhibit symptoms such as pollakiuria (p < 0.001) or dysuria (p = 0.006) at the time of enrollment (Table 2). However, following treatment, some children presented clinical signs, although the differences were not statistically significant (p > 0.05).
Cure and egg reduction rates according to demographic factors
S. haematobium infection
The overall cure rate (CR) for Schistosoma haematobium was 80.5% (62/77), which falls below the WHO efficacy threshold of 90% for praziquantel. Similarly, the overall egg reduction rate (ERR) was 87.3%, also below the WHO-recommended level of ≥95%. A significantly higher cure rate (CR) was observed among children in the univariate analysis according to study sites (p < 0.0001); however, the multivariate analysis showed an OR of 1.4 (95%CI: 0.00-5.80) in favor of Taliko (p > 0.9). In Taliko, the highest CR (79.4%) was recorded among children with low infection intensity, with an ERR of 83.8%. In Missabougou, all children (100%) with low baseline egg loads achieved complete parasitological clearance two weeks after treatment. ERRs were 89.5% and 83.8% among participants with high and low egg burdens in Taliko, respectively. When stratified by sex, CRs were 83.3% in females and 78.0% in males, a non-significant difference (p = 0.89). ERRs were slightly higher in females (90.3%) than in males (86.0%). The stratified analysis of cure rates (CR) according to infection intensity across different age groups showed that CRs varied significantly between age categories (p < 0.0001). Moreover, the overall CR was higher among younger children (6–10 years) compared to older ones (11–14 years), with 90.9% and 78.8%, respectively (OR=0.35; 95%CI: 0.02-2.15; p=0.3). (Tables 3 & 4). Although efficacy in younger children approached WHO standards, results in older children remained suboptimal.
S. mansoni infection
Among the 35 stool samples positive for S. mansoni and re-examined two weeks after treatment, the overall CR was 91.4% (32/35), which meets the WHO efficacy threshold (≥90%), and the ERR reached 98%, above the WHO-recommended level (≥95%). A statistically significant difference in CR was found between study sites at univariate anlysis, ranging from 77.8% in Missabougou to 96.2% in Taliko (p = 0.002). Both CR and ERR were higher among participants with high baseline egg counts compared to those with lower intensities. ERRs were relatively consistent between sites but tended to be higher in heavily infected individuals. Sex-stratified analysis indicated that girls in Taliko had a higher proportion of heavy infections (64.8%), whereas boys were predominantly lightly infected (61.3%). The CRs were 88.2% in females and 94.4% in males; the difference was not statistically significant at univariate analysis (p = 0.86). ERRs were slightly higher in females (98.8%) than in males (97.1%), both exceeding WHO efficacy standards. Among girls, the highest ERR (100%) was observed in those with low infection intensity, whereas among boys, it was observed in those with moderate infection intensity. Among children aged 6–10 years, low-intensity infections predominated (57.1%), with no moderate infections detected. In contrast, 50.0% of those aged 11–14 years had high infection intensities. The overall CR across age groups was 85.7%, showing not significant variation between age categories at bivariate analysis (OR=0.95, 95%CI: 0.04-13.5, p>0.9). The highest CR was recorded among low-intensity infections in younger children, while in older children, higher CRs were observed among those with heavier infections. Overall ERRs were 98.9% in younger children and 97.8% in older children, both above the WHO-recommended threshold (Tables 5 & 6).
Of the 99 children re-evaluated two weeks after praziquantel administration, 13 (13.1%) were coinfected with S. haematobium and S. mansoni, all residing in Taliko. The overall CR among coinfected children was 69.2%, which is substantially below the WHO-recommended level of ≥90%, suggesting a possible reduced efficacy of praziquantel in mixed infections. No statistically significant difference in CR was observed between sexes (OR=1.30; 95%CI: 0.15-14.9, p=0.8). All coinfected individuals belonged to the 11–14-year age group.
Discussion
This study aimed to assess the efficacy of praziquantel (PZQ) in school-aged children after more than a decade of mass drug administration. A large proportion of infected children reported frequent contact with natural water bodies, underscoring the critical role of water sources in the transmission dynamics of schistosomiasis. These findings are consistent with those of Agniwo et al. (2023) [9], who reported in the Kayes region of Mali that parental occupation had a modest influence on infection prevalence, with higher infection rates observed among children of traders and fishermen. The association between water contact and infection is likely attributable to the participation of children in high-risk activities such as swimming, recreational play, and other behaviours that increase exposure to contaminated water.
Clinically, the most frequently reported symptoms among Schistosoma haematobium-infected children were bladder and abdominal pain, which were significantly more common than in non-infected children, followed by dysuria and pollakiuria. These symptoms showed notable improvement within two weeks following PZQ treatment. The prevalence of abdominal pain (66.7%) and dysuria (46.7%) in the present study was higher than that previously reported in the Kayes region in 2021 (46.1% and 39.6%, respectively), whereas the frequency of pollakiuria (33.3%) was lower compared to the 58.4% reported in Kayes [9].
The ERR was calculated in addition to the CR, as Montresor (2011) suggested that the CR alone was not a valid indicator for assessing drug efficacy [22]. According to WHO guidelines, a satisfactory efficacy is defined by a cure rate (CR) and egg reduction rate (ERR) of at least 90%. Based on these criteria, praziquantel efficacy against S. haematobium in this study (CR = 80.5%; ERR = 87.3%) would be considered “doubtful efficacy”, whereas efficacy against S. mansoni (CR = 91.4%; ERR = 98.0%) meets the WHO definition of “satisfactory efficacy”. The lower response observed in co-infected children (CR = 69.2%) would correspond to “reduced efficacy”.
This difference in CR and ERR by species could be explained by their incubation times, since reinfection with S. mansoni generally occurs more rapidly than with S. haematobium, as observed in several studies of mixed infections [23,24]. The differential efficacy of praziquantel observed between S. haematobium and S. mansoni could possibly be related to the migratory behavior of mature S. mansoni worms, as suggested by previous studies, although this mechanism remains hypothetical and requires experimental confirmation. Further parasitological or pharmacokinetic studies would be required to substantiate this hypothesis. The low cure rates observed in this study, particularly in Taliko, may reflect ongoing transmission, although longitudinal data are needed to confirm this. This finding may suggest the need to reconsider the current dosing regimen or the possibility of emerging parasite resistance. Specifically, aged S. mansoni adults are transported from the mesenteric venous plexus through the portal vein to the liver, where they can undergo a period of recovery before migrating back to their oviposition sites. This hepatic sequestration may reduce drug susceptibility and contribute to the reduced praziquantel efficacy against S. mansoni [25]. Praziquantel remains the drug of choice for schistosomiasis treatment, pending the development of a more effective single-dose alternative [21]. Despite sustained selective pressure since its introduction as an antiparasitic agent in 1975, praziquantel continues to exhibit a favorable safety and tolerability profile, with adverse effects typically being mild, transient, and resolving within one week post-treatment [26]. Accordingly, the World Health Organization (WHO) recommends large-scale preventive chemotherapy through periodic mass administration of single-dose praziquantel to all at-risk populations in endemic regions as the cornerstone strategy for schistosomiasis control and elimination. Given emerging concerns, rigorous monitoring of treatment efficacy is imperative, particularly as previous studies have documented reductions in praziquantel efficacy, evidenced by egg reduction rates (ERR) below 90% and suboptimal cure rates (CR) following treatment [27]. The overall cure rate (80.5%) and egg reduction rate (87.3%) for S. haematobium therefore correspond to the WHO-defined category of “doubtful efficacy” (80–90%), suggesting a possible decline in treatment performance that warrants further monitoring. Conversely, numerous other investigations have reported satisfactory CRs, including a multicenter trial assessing the therapeutic efficacy of a single 40 mg/kg oral dose of praziquantel against Schistosoma mansoniand S. haematobium infections across six African countries (Cameroon, Ethiopia, Mali, Madagascar, Tanzania, and Zanzibar), as well as against S. mansoni in Brazil and S. japonicum in the Philippines [28,29].
The cure rate (CR) for Schistosoma haematobium observed in our study (80.5%; Figure 3) was higher than that reported by Tchuenté et al. (2004) in Loum, Cameroon, where a CR of 50.4% was recorded three weeks post-treatment [30]. Similarly, in Niger, children infected with S. haematobium exhibited a CR of 53.1% and an egg reduction rate (ERR) of 84.9% six weeks after treatment with 40 mg/kg praziquantel [31]. In Nigeria, a CR of 49.4% was reported at four weeks post-treatment [32], however, these authors documented CRs comparable to our findings nine months after treatment [33]. A study conducted in south-central Côte d’Ivoire reported an acceptable CR of 82% four weeks post-treatment with a single 40 mg/kg dose of praziquantel, consistent with our results [34]. Higher CRs of 96% and 93% have been reported in Nigeria and Côte d’Ivoire, respectively [35,36]. Similarly, a recent study conducted in several villages along the Senegal River Basin on urogenital schistosomiasis reported CRs ranging from 96.5% to 98.0% in lakeside villages and 88.5% in a canal-irrigated village, with ERRs ranging from 96.7% to 99.7% [37]. The relatively low CRs observed in the present study, particularly in Taliko, may reflect ongoing transmission or early reinfections, although longitudinal data would be required to confirm this. Moreover, differences between study sites should be interpreted cautiously due to variation in sample size and potential unmeasured environmental or behavioral confounders.
For Schistosoma mansoni, the cure rate (CR) of 91.4% and egg reduction rate (ERR) of 98.0% observed in our study were significantly higher than those reported in the Senegal River Valley, where a CR of 76.1% was recorded following two 40 mg/kg doses of praziquantel administered over four weeks [38]. Similarly, in Niger, lower CR (59.6%) and treatment response outcomes (TRO) of 56.1% were reported at six weeks post-treatment with 40 mg/kg praziquantel [31]. Webster et al. (2013) reported CRs ranging from 81% to 95.5%, accompanied by high ERRs of 98.4% to 98.9%, comparable to our findings, though assessments were conducted six weeks after treatment [39]. These authors hypothesized that the persistence of S. mansoni infection in the village of Nder may result from rapid reinfection dynamics or suboptimal praziquantel efficacy in this setting.
The cure and egg reduction rates in Missabougou (17 out of 99), being below 90%, would fall into the WHO-defined category of “reduced efficacy” although interpretation is limited by small sample size (Figure 3). This reduced prevalence may partly reflect recent environmental changes that are unfavorable to the parasite’s lifecycle; however, this hypothesis cannot be confirmed in the absence of specific environmental data [20]. Consequently, this explanation remains speculative until corroborated by ecological or malacological assessments. Nevertheless, the small sample size of treated individuals in this locality necessitates prudence when extrapolating these findings. Indeed, the relatively small number of children included, particularly in Missabougou, reduces the statistical power of the analyses and limits the generalization of the findings to the entire exposed population. This constraint may partly explain the low prevalence of schistosomiasis observed in Missabougou (an urban area) and the resulting variability in cure rates (CR) and egg reduction rates (ERR). Furthermore, the loss to follow-up recorded after treatment administration represents another potential source of bias across the study sites. It is likely that the children lost to follow-up had different exposure patterns, infection intensities, or treatment responses compared to those who were reassessed, thereby introducing a selection bias that could either overestimate or underestimate the true efficacy of praziquantel across the different study.
The observed variation in cure rate (CR) and egg reduction rate (ERR) according to age suggests a possible trend toward greater efficacy of praziquantel among younger children. However, this observation should be interpreted with caution given the limited size of the subgroups. Further studies involving larger, age-stratified cohorts are needed to confirm this trend. Other studies have attributed higher CRs in older children to the higher absolute doses of PZQ administered, as dosing is weight-dependent [26]. Considerable variation in PZQ efficacy has also been reported relative to infection intensity. While our study demonstrated variable CRs and ERRs across different intensity levels, research from Ethiopia indicated that individuals with moderate to high egg burdens exhibited relatively elevated ERRs at various follow-up intervals. Although subgroup analysis by infection intensity provided useful insights, these comparisons were based on small numbers and should therefore be interpreted as indicative rather than conclusive. Conversely, in Mozambique, PZQ efficacy was found to be inversely correlated with parasite load [40]. Importantly, previous investigations have suggested that reductions in egg excretion post-treatment may result from praziquantel’s impact on parasite fecundity rather than immediate worm elimination, thereby accounting for the high ERRs observed [41].
Variations in reported cure rates (CRs) may result from multiple factors beyond ecological and epidemiological conditions, such as the presence of temporary versus permanent water bodies, patterns of human water contact, pre-treatment infection intensity, and schistosome species diversity [24,42]. Additionally, the potential emergence of anthelmintic resistance, as outlined by the WHO (2013), may compromise praziquantel efficacy. For instance, continued egg excretion post-treatment could reflect residual egg release from deceased worms, or administration of praziquantel during the immature worm stages, against which the drug is known to be less effective [43]. Although hybrid schistosomes (S. haematobium × S. bovis and S. haematobium × S. curassoni) have been reported in Mali [9] and neighboring countries such as Senegal [44] and Niger [45], suggesting possible implications for praziquantel susceptibility, no genotyping or egg viability analyses were conducted in the present study. Therefore, the mention of potential hybridization as a factor influencing drug efficacy should be interpreted with caution. Further molecular investigations, including genetic characterization of miracidia or eggs, are needed to confirm the presence of hybrid parasites and to better understand their epidemiological and therapeutic significance. Preventive chemotherapy (PTC) is also hypothesized to induce genetic alterations in schistosome populations driven by drug-induced selection pressures [46]. Comparable phenomena have been observed with ivermectin treatment of Onchocerca volvulus, resulting in reduced female parasite fecundity [47]. However, no genetic data were collected in this study, and such evolutionary interpretations remain conjectural.
ERR values were categorized as follows: satisfactory (ERR ≥ 90%), doubtful (90% > ERR ≥ 80%), and reduced efficacy (ERR ≤ 80%). The cure rate (CR) among children co-infected with S. haematobium and S. mansoni was 69.2%, lower than the 97.1% reported by Garba (2013). In our cohort, most co-infected children exhibited higher baseline egg counts for S. haematobium than for S. mansoni, suggesting that S. haematobium may have contributed more strongly to the overall lower cure rate observed in mixed infections. However, the small number of co-infection cases did not allow a robust comparison of species-specific therapeutic responses. It is therefore possible that both species contributed to the reduced efficacy observed, considering potential interspecific interactions and differences in praziquantel susceptibility. The relationship between mixed-species infection and infection intensity likely varies according to differences in local transmission dynamics and the endemicity of each schistosome species [48]. Further site-specific investigations are needed to clarify whether these interactions affect the response to praziquantel.
Finally, it is important to note that the potential bias related to reinfection was minimized in this study by assessing parasitemia 14 days after treatment [21]. Overall, according to WHO efficacy benchmarks, the results obtained in this study indicate satisfactory efficacy against S. mansoni, doubtful efficacy against S. haematobium, and reduced efficacy in cases of co-infection. Therefore, the results presented here should be interpreted with caution, considering the limited sample size, the short follow-up period, and the absence of long-term parasitological confirmation.
Conclusion
The results of this study suggest that praziquantel (PZQ) may be highly effective in treating school-aged children infected with Schistosoma mansoni in Taliko and Missabougou, within the Bamako district; however, these findings should be interpreted with caution and confirmed through further studies. However, a lower efficacy of PZQ was observed against S. haematobium, highlighting the need for further follow-up studies in Mali. These should include egg viability assessments after treatment and systematic monitoring of drug efficacy within the framework of the National Schistosomiasis Control Program (PNLSH). Beyond the potential for parasite tolerance to PZQ, the persistence of schistosomiasis in the Bamako district also underlines the importance of complementary control measures, such as health education in schools and environmental sanitation along the banks of the Niger River and its tributaries the main snail breeding sites to help interrupt transmission. Furthermore, continued efforts to identify and develop new molecules to complement the action of PZQ remain essential in the context of parasite evolution.
What is already known about the topic
- Schistosomiasis is a neglected parasitic disease caused by Schistosoma spp., transmitted through contact with freshwater infested with cercariae.
- Despite large-scale control efforts using praziquantel (PZQ, 40 mg/kg), the disease remains endemic in many sub-Saharan African countries, particularly Mali.
- PZQ is effective in reducing parasite burden, but its efficacy varies with species, infection intensity, age, geography, and water contact behaviors.
- Continued transmission in some areas[28,29], despite repeated treatment campaigns, raises concerns about possible reduced efficacy or rapid reinfection. These observations underscore the importance of ongoing monitoring of treatment effectiveness and transmission dynamics to support schistosomiasis control and elimination efforts.
What this study adds
- This study provides updated evidence on praziquantel (PZQ) efficacy in school-aged children infected with Schistosoma haematobiumand mansoni in urban and peri-urban areas of Bamako, Mali.
- While PZQ showed overall satisfactory efficacy, cure rates (CR) and egg reduction rates (ERR) were below WHO-recommended thresholds in some cases, especially in coinfections or high-intensity infections.
- The study highlights variations in response by site, age, sex, and socioeconomic status, and presents the first data suggesting reduced PZQ efficacy in West Africa.
- It underscores the role of repeated water contact and calls for adapted control strategies and ongoing efficacy surveillance in endemic areas.
Acknowledgements
The authors express their gratitude to the principals of the participating schools and the schoolchildren who provided biological samples. Appreciation is also extended to the staff of the Malaria Research and Training Center (MRTC), as well as to the personnel of the Faculties of Medicine and Dentistry and Pharmacy for facilitating the laboratory work. We thank Dr. Mahamane Maîga for his support in data analysis.
Authors´ contributions
P.A., S.N.D., A.D., D.L, AD, BT, MT, A.K, A.A.D. participated in the design of the study, updated the research methodology, results validation and contributed to the writing of the final document. P.A., A.D., S.N.D., A.A., H.G., OC, O.D, AK, AG, R.D, coordinated the field trial. P.A., A.D, and S.N.D. carried out the statistical analysis. All authors reviewed the manuscript for submission.
| Variables | Frequency (n=99) | Percentage (%) |
|---|---|---|
| Sites | ||
| Taliko | 82 | 82.8 |
| Missabougou | 17 | 17.2 |
| Gender | ||
| Male | 52 | 52.5 |
| Female | 47 | 47.5 |
| Age | ||
| 6–10 | 18 | 18.2 |
| 11–14 | 81 | 81.8 |
| Taking traditional medicines | ||
| No | 61 | 61.6 |
| Yes | 38 | 38.4 |
| River frequentation | ||
| Yes | 77 | 77.8 |
| No | 22 | 22.2 |
| Habitat–River Distance | ||
| < 50 m | 94 | 94.9 |
| ≥ 50 m | 5 | 5.1 |
| Domestic water source | ||
| River | 1 | 1 |
| Well | 1 | 1 |
| Tap | 82 | 82.8 |
| Borehole | 15 | 15.2 |
| Parents profession | ||
| Shopkeeper | 10 | 10.1 |
| Civil servant | 5 | 5.1 |
| Gardener | 1 | 1 |
| Manual labor | 82 | 82.8 |
| Fisherman | 1 | 1 |
| S. haematobium | ||
| Yes | 77 | 77.8 |
| No | 22 | 22.2 |
| S. mansoni | ||
| Yes | 35 | 35.4 |
| No | 64 | 64.6 |
| Coinfected | ||
| Yes | 13 | 13.1 |
| No | 86 | 86.9 |
| Clinical signs | Pre-treatment n=77 (%) | p | Post-treatment n=15 (%) | p |
|---|---|---|---|---|
| Bladder pain | ||||
| No | 36 (46.8) | 0.65 | 8 (53.3) | >0.05 |
| Yes | 41 (53.2) | 7 (46.7) | ||
| Abdominal pain | ||||
| No | 32 (41.6) | 0.17 | 5 (33.3) | 0.30 |
| Yes | 45 (58.4) | 10 (66.7) | ||
| Pollakiuria | ||||
| No | 58 (75.3) | <0.001 | 10 (66.7) | 0.30 |
| Yes | 19 (24.7) | 5 (33.3) | ||
| Dysuria | ||||
| No | 51 (66.2) | 0.006 | 8 (53.3) | >0.05 |
| Yes | 26 (33.8) | 7 (46.7) |
| Demographic variable | Number Examined (%) | Number cured (Cure Rate) | 95% CI | p | AEMC before treatment | AEMC after treatment | ERR (%) | |
|---|---|---|---|---|---|---|---|---|
| Sites | ||||||||
| Taliko | Low | 63 (91.3) | 50 (79.4) | [68.4–88.1] | <0.0001 | 5 | 7 | 83.8 |
| High | 6 (8.7) | 4 (66.7) | [22.3–95.7] | 72 | 0 | 89.5 | ||
| Total | 69 | 54 (78.3) | [68.4–88.1] | 87.0 | ||||
| Missabougou | Low | 8 | 8 (100.0) | [63.1–100] | 2 | 0 | 100 | |
| High | 0 | – | – | – | – | – | ||
| Total | 8 (100.0) | 8 (100.0) | [63.1–100] | 100 | ||||
| Gender | ||||||||
| Female | Low | 36 (100) | 30 (83.3) | [71.1–95.5] | 0.89 | 6 | 4 | 90.3 |
| High | 0 | – | – | – | – | – | ||
| Total | 36 | 30 (83.3) | [71.1–95.5] | 90.3 | ||||
| Male | Low | 35 (85.4) | 28 (80) | [63.9–91.6] | 4 | 9 | 74.4 | |
| High | 6 (14.6) | 4 (66.7) | [22.3–95.7] | 72 | – | 89.5 | ||
| Total | 41 | 32 (78.0) | [65.5–90.5] | 86.0 | ||||
| Age (years) | ||||||||
| 6–10 | Low | 10 (90.0) | 9 (90) | [55.5–99.7] | <0.0001 | 7 | 2 | 96.4 |
| High | 1 (10.0) | 1 (100) | [2.5–100] | 52 | – | 100 | ||
| Total | 11 | 10 (90.9) | [74.1–100] | 98.1 | ||||
| 11–14 | Low | 61 (92.4) | 49 (80.3) | [68.8–88.9] | 5 | 7 | 82.3 | |
| High | 5 (7.6) | 3 (60) | [14.7–94.7] | 75 | – | 88.1 | ||
| Total | 66 | 52 (78.8) | [69.0–88.6] | 85.5 | ||||
| Total | 77 | 62 (80.5) | [71.2–89.8] | 87.3 | ||||
| Demographic variable | Subcategory | OR | 95% CI | p-value |
|---|---|---|---|---|
| Site | Missabougou | — | — | — |
| Taliko | 1.4 | 0.00–5.80 | >0.9 | |
| Gender | Female | — | — | — |
| Male | 1.76 | 0.55–5.95 | 0.3 | |
| Age (years) | 6–10 | 0.35 | 0.02–2.15 | 0.3 |
| 11–14 | — | — | — |
| Demographic variable | Subcategory | Number Examined (%) | Number cured (CR) | 95% CI | p | AEMC before treatment | AEMC after treatment | ERR (%) |
|---|---|---|---|---|---|---|---|---|
| Site | ||||||||
| Taliko | Low | 6 (23.1) | 5 (83.3) | [35.9 – 99.6] | 0,002 | 72 | 84 | 39 |
| Moderate | 4 (15.4) | 3 (75) | [19.4 – 99.4] | 0,002 | 354 | 264 | 91.5 | |
| High | 16 (61.5) | 15 (93.8) | [69.8 – 99.8] | 0,002 | 1512 | – | 99.8 | |
| Total | 26 | 25 (96.2) | [80.4 – 99.9] | 0,002 | – | – | 98.3 | |
| Missabougou | Low | 8 (88.9) | 6 (75) | [34.9 – 96.8] | 0,002 | 51 | 60 | 70.6 |
| Moderate | 0 | – | [2.5 – 100] | 0,002 | – | – | – | |
| High | 1 (11.1) | 1 (100) | [40.0 – 97.2] | 0,002 | 1224 | – | 100 | |
| Total | 9 | 7 (77.8) | [35.9 – 99.6] | 0,002 | – | – | 92.6 | |
| Gender | ||||||||
| Female | Low | 3 (17.6) | 3 (100) | [29.2 – 100] | 0,86 | 56 | 84 | 100 |
| Moderate | 3 (17.6) | 2 (66.7) | [9.4 – 99.2] | 0,86 | 344 | – | 88.4 | |
| High | 11 (64.8) | 10 (90.9) | [58.7 – 99.8] | 0,86 | 1210 | – | 99.6 | |
| Total | 17 | 15 (88.2) | [63.6 – 98.5] | 0,86 | – | – | 98.8 | |
| Male | Low | 11 (61.2) | 8 (72.7) | [39.0 – 93.9] | 0,86 | 61 | 60 | 42.9 |
| Moderate | 1 (5.5) | 1 (100) | [2.5 – 100] | 0,86 | 384 | 264 | 100 | |
| High | 6 (33.3) | 6 (100) | [54.1 – 100] | 0,86 | 2016 | – | – | |
| Total | 18 | 17 (94.4) | [72.7 – 99.9] | 0,86 | – | – | 97.1 | |
| Age (years) | ||||||||
| 6-10 | Low | 4 (57.1) | 4 (100) | [39.8 – 100] | 0,0005 | 66 | 48 | 100 |
| Moderate | 0 | – | [9.4 – 99.2] | 0,0005 | – | – | – | |
| High | 3 (42.9) | 2 (66.7) | [42.1 – 99.6] | 0,0005 | 1344 | – | 98.8 | |
| Total | 7 | 6 (85.7) | [34.8 – 93.3] | 0,0005 | – | – | 98.9 | |
| 11-14 | Low | 10 (35.7) | 7 (70) | [19.4 – 99.4] | 0,0005 | 58 | 80 | 33.3 |
| Moderate | 4 (14.3) | 3 (75) | [76.8 – 100] | 0,0005 | 354 | 264 | 91.5 | |
| High | 14 (50.0) | 14 (100) | [67.3 – 96.0] | 0,0005 | 1527 | – | 100 | |
| Total | 28 | 24 (85.7) | [76.9 – 98.2] | 0,0005 | – | – | 97.8 | |
| Total | 35 | 30 (85.7) | [39.8 – 100] | – | – | – | 98 | |
| Demographic variable | Subcategory | OR | 95% CI | p-value |
|---|---|---|---|---|
| Site | Missabougou | — | — | — |
| Taliko | 0.48 | 0.06, 4.71 | 0.5 | |
| Gender | Female | — | — | — |
| Male | 1.30 | 0.15, 14.9 | 0.8 | |
| Age (years) | 6-10 | 0.95 | 0.04, 13.5 | >0.9 |
| 11-14 | — | — | — |



References
- Dembélé M, Bamani S, Dembélé R, Traoré MO, Goita S, Traoré MN, Sidibe AK, Sam L, Tuinsma M, Toubali E, MacArthur C, Baker SK, Zhang Y. Implementing preventive chemotherapy through an integrated national neglected tropical disease control program in Mali. Lammie PJ, editor. PLoS Negl Trop Dis. 2012;6(3):e1574. doi: 10.1371/journal.pntd.0001574. Available from: https://dx.plos.org/10.1371/journal.pntd.0001574.
- Atalabi TE, Adoh SD, Eze KM. The current epidemiological status of urogenital schistosomiasis among primary school pupils in Katsina State, Nigeria: an imperative for a scale up of water and sanitation initiative and mass administration of medicines with Praziquantel. Gray DJ, editor. PLoS Negl Trop Dis. 2018;12(7):e0006636. doi: 10.1371/journal.pntd.0006636. Available from: https://dx.plos.org/10.1371/journal.pntd.0006636.
- World Health Organization. Schistosomiasis and soil-transmitted helminthiases: progress report, 2021. Wkly Epidemiol Rec. 2022;97(48):621-632. Available from: https://www.who.int/publications/i/item/who-wer9748-621-632.
- Dawet A. Prevalence and intensity of Schistosoma haematobium among residents of Gwong and Kabong in Jos North Local Government Area, Plateau State, Nigeria. Int J Biol Chem Sci. 2012;6(4):1557-1565. doi: 10.4314/ijbcs.v6i4.15. Available from: https://www.ajol.info/index.php/ijbcs/article/view/84002.
- Doumenge JP, Mott KE, Cheung C, Villenave D, Chapuis O, Perrin MF, Reaud-Thomas G. Atlas of the global distribution of schistosomiasis. Talence: Presses Universitaires de Bordeaux; 1987. 400 p.
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368(9541):1106-1118. doi: 10.1016/S0140-6736(06)69440-3. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673606694403.
- Clements ACA, Bosqué-Oliva E, Sacko M, Landouré A, Dembélé R, Traoré M, Coulibaly G, Gabrielli AF, Fenwick A, Brooker S. A comparative study of the spatial distribution of schistosomiasis in Mali in 1984–1989 and 2004–2006. Seto EYW, editor. PLoS Negl Trop Dis. 2009;3(5):e431. doi: 10.1371/journal.pntd.0000431. Available from: https://dx.plos.org/10.1371/journal.pntd.0000431.
- Bintou LY, Yaro AS, Sodio B, Sacko M. Persistance de la schistosomiase urinaire en zones endémiques soumises aux traitements de masse répétés au Mali. Int J Biol Chem Sci. 2019;13(1):369-381. Available from: https://www.ajol.info/index.php/ijbcs/article/view/186741.
- Agniwo P, Sidibé B, Diakité A, Niaré SD, Guindo H, Akplogan A, Ibikounlé M, Boissier J, Dabo A. Ultrasound aspects and risk factors associated with urogenital schistosomiasis among primary school children in Mali. Infect Dis Poverty. 2023;12(1):40. doi: 10.1186/s40249-023-01071-6. Available from: https://idpjournal.biomedcentral.com/articles/10.1186/s40249-023-01071-6.
- Coulibaly BM. La schistosomiase tissulaire au Mali: à propos de 145 cas au service d’anatomie et cytologie pathologiques [dissertation]. Bamako: Université des Sciences, des Techniques et des Technologies de Bamako; 2012. 72 p. Available from: https://www.bibliosante.ml/bitstream/handle/123456789/1544/13M101.pdf?sequence=1.
- World Health Organization. Intégrer les maladies tropicales négligées dans l’action pour la santé mondiale et le développement: quatrième rapport de l’OMS sur les maladies tropicales négligées. Geneva: WHO; 2018. 271 p. French. Available from: https://apps.who.int/iris/bitstream/handle/10665/260289/9789242565447-fre.pdf?sequence=1.
- World Health Organization. WHO guideline on control and elimination of human schistosomiasis. Geneva: WHO; 2022 Feb 15. 118 p. Available from: https://www.ncbi.nlm.nih.gov/books/NBK578392/pdf/Bookshelf_NBK578392.pdf.
- World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. Geneva: WHO; 2002. 57 p. (WHO Technical Report Series, No. 912). Available from: https://www.who.int/publications/i/item/WHO-TRS-912.
- Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, Zhang Y, Garba A, Stothard JR, Gabrielli AF, Clements ACA, Kabatereine NB, Toure S, Dembele R, Nyandindi U, Mwansa J, Koukounari A. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology. 2009;136(13):1719-1730. doi: 10.1017/S0031182009990400. Available from: https://www.cambridge.org/core/journals/parasitology/article/abs/schistosomiasis-control-initiative-sci-rationale-development-and-implementation-from-20022008/….
- Stothard JR, Webster BL, Weber T, Nyakaana S, Webster JP, Kazibwe F, Kabatereine NB, Rollinson D. Molecular epidemiology of Schistosoma mansoni in Uganda: DNA barcoding reveals substantial genetic diversity within Lake Albert and Lake Victoria populations. Parasitology. 2009;136(13):1813-1824. doi: 10.1017/S003118200999031X. Available from: https://www.cambridge.org/core/journals/parasitology/article/abs/molecular-epidemiology-of-schistosoma-mansoni-in-uganda-dna-barcoding-reveals-substantial-genetic-diversity-within-lake-albert-and-lake-victoria-populations/….
- Ernould JC, Ba K, Sellin B. Increase of intestinal schistosomiasis after praziquantel treatment in a Schistosoma haematobium and Schistosoma mansoni mixed focus. Acta Trop. 1999;73(2):143-152. doi: 10.1016/S0001-706X(99)00013-3. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0001706X99000133.
- Koukounari A, Donnelly CA, Sacko M, Keita AD, Landouré A, Dembelé R, Bosqué-Oliva E, Gabrielli AF, Gouvras A, Traoré M, Fenwick A, Webster JP. The impact of single versus mixed schistosome species infections on liver, spleen and bladder morbidity within Malian children pre- and post-praziquantel treatment. BMC Infect Dis. 2010;10:227. doi: 10.1186/1471-2334-10-227. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-10-227.
- Dabo A, Diarra AZ, Machault V, Touré O, Niambélé DS, Kanté A, Ongoiba A, Doumbo O. Urban schistosomiasis and associated determinant factors among school children in Bamako, Mali, West Africa. Infect Dis Poverty. 2015;4:4. doi: 10.1186/2049-9957-4-4. Available from: https://idpjournal.biomedcentral.com/articles/10.1186/2049-9957-4-4.
- Tandina F, Doumbo SN, Koné AK, Guindo D, Goita S, Sissoko M, Konaté S, Dabo A, Doumbo OK. Épidémiologie de la schistosomiase dans la zone périurbaine de Sotuba, 10 ans après le début des traitements de masse au Mali. Med Sante Trop. 2016;26(1):51-56. doi: 10.1684/mst.2015.0515. Available from: https://www.jle.com/fr/revues/mst/e-docs/epidemiologie_de_la_schistosomiase_dans_la_zone_periurbaine_de_sotuba_10_ans_apres_le_debut_des_traitements_de_masse_au_mali_305540/article.phtml. French.
- Seck MC, Gueye PAT, Faye C, Engo PE, Diongue K, Ndiaye M, Badiane AS, Ndiaye D. Trichomonas vaginalis and Mycoplasma co-infection among women received in a microbiology laboratory in Dakar. Afr J Pathol Microbiol Epidemiol. 2024;2(1):0. doi: 10.35995/ajpme2010003. Available from: https://ajpme.jams.pub/article/2/1/270.
- World Health Organization. Assessing the efficacy of anthelminthic drugs against schistosomiasis and soil-transmitted helminthiases. Geneva: WHO; 2013. 39 p. (WHO/HTM/NTD/PCT/2013.4). Available from: https://www.who.int/publications/i/item/9789241564557.
- Montresor A. Cure rate is not a valid indicator for assessing drug efficacy and impact of preventive chemotherapy interventions against schistosomiasis and soil-transmitted helminthiasis. Trans R Soc Trop Med Hyg. 2011;105(7):361-363. doi: 10.1016/j.trstmh.2011.04.003. Available from: https://academic.oup.com/trstmh/article/105/7/361/1862344.
- Daffalla AA, Fenwick A. Resurgence of Schistosoma mansoni and Schistosoma haematobium after the end of a 4-year control programme against S. mansoni. Trans R Soc Trop Med Hyg. 1982;76(5):701-702. doi: 10.1016/0035-9203(82)90245-0. Available from: https://academic.oup.com/trstmh/article/76/5/701/1862345.
- Ernould JC, Garba A, Labbo R, Kaman Kaman A, Sidiki A, Djibrilla A, Chippaux JP. Hétérogénéité de la transmission de Schistosoma haematobium dans les périmètres irrigués du Niger. Bull Soc Pathol Exot. 2004;97(1):19-23. Available from: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers12-10/010033833.pdf. French.
- Omer AHS, Teesdale CH. Metrifonate trial in the treatment of various presentations of Schistosoma haematobium and S. mansoni infections in the Sudan. Ann Trop Med Parasitol. 1978;72(2):145-150. doi: 10.1080/00034983.1978.11719296. Available from: https://www.tandfonline.com/doi/abs/10.1080/00034983.1978.11719296.
- Gebreyesus TD, Makonnen E, Tadele T, Gashaw H, Degefe W, Gerba H, Tadesse BT, Gurumurthy P, Aklillu E. Safety surveillance of mass praziquantel and albendazole co-administration in school children from Southern Ethiopia: an active cohort event monitoring. J Clin Med. 2022;11(21):6300. doi: 10.3390/jcm11216300. Available from: https://www.mdpi.com/2077-0383/11/21/6300.
- Utzinger J, N’Goran EK, N’Dri A, Lengeler C, Tanner M. Efficacy of praziquantel against Schistosoma mansoni with particular consideration for intensity of infection. Trop Med Int Health. 2000;5(11):771-778. doi: 10.1046/j.1365-3156.2000.00646.x. Available from: https://onlinelibrary.wiley.com/doi/10.1046/j.1365-3156.2000.00646.x.
- Tchuem Tchuenté LA, Momo SC, Stothard JR, Rollinson D. Efficacy of praziquantel and reinfection patterns in single and mixed infection foci for intestinal and urogenital schistosomiasis in Cameroon. Acta Trop. 2013;128(2):275-283. doi: 10.1016/j.actatropica.2013.06.007. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0001706X1300154X.
- Levecke B, Vlaminck J, Andriamaro L, Ame S, Belizario V, Degarege A, Engels D, Erko B, Garba AD, Kaatano GM, Mekonnen Z, Montresor A, Olliaro P, Pieri OS, Sacko M, Sam-Wobo SO, Tchuem Tchuenté LA, Webster JP, Vercruysse J. Evaluation of the therapeutic efficacy of praziquantel against schistosomes in seven countries with ongoing large-scale deworming programs. Int J Parasitol Drugs Drug Resist. 2020;14:183-187. doi: 10.1016/j.ijpddr.2020.10.003. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2211320720300348.
- Mekonnen A, Legesse M, Belay M, Tadesse K, Torben W, Teklemariam Z, Erko B. Efficacy of praziquantel against Schistosoma haematobium in Dulshatalo village, western Ethiopia. BMC Res Notes. 2013;6:392. doi: 10.1186/1756-0500-6-392. Available from: https://bmcresnotes.biomedcentral.com/articles/10.1186/1756-0500-6-392.
- Garba A, Lamine MS, Barkiré N, Djibo A, Sofo B, Gouvras AN, Labbo R, Sebangou H, Webster JP, Fenwick A, Utzinger J. Efficacy and safety of two closely spaced doses of praziquantel against Schistosoma haematobium and S. mansoni and re-infection patterns in school-aged children in Niger. Acta Trop. 2013;128(2):334-344. doi: 10.1016/j.actatropica.2012.08.008. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0001706X1200280X.
- Ojurongbe O, Sina-Agbaje OR, Busari A, Okorie PN, Ojurongbe TA, Akindele AA. Efficacy of praziquantel in the treatment of Schistosoma haematobium infection among school-age children in rural communities of Abeokuta, Nigeria. Infect Dis Poverty. 2014;3:30. doi: 10.1186/2049-9957-3-30. Available from: https://idpjournal.biomedcentral.com/articles/10.1186/2049-9957-3-30.
- Tchuenté LAT, Shaw DJ, Polla L, Cioli D, Vercruysse J. Efficacy of praziquantel against Schistosoma haematobium infection in children. Am J Trop Med Hyg. 2004;71(6):778-782. Available from: https://pubmed.ncbi.nlm.nih.gov/15642971/.
- N’Goran EK, Utzinger J, N’Guessan AN, Müller I, Zamblé K, Lohourignon KL, Traoré M, Sosthène BA, Lengeler C, Tanner M. Reinfection with Schistosoma haematobium following school-based chemotherapy with praziquantel in four highly endemic villages in Côte d’Ivoire. Trop Med Int Health. 2001;6(10):817-825. doi: 10.1046/j.1365-3156.2001.00785.x. Available from: https://onlinelibrary.wiley.com/doi/10.1046/j.1365-3156.2001.00785.x.
- Houmsou RS, Wama BE, Agere H, Uniga JA, Amuta EU, Kela SL. High efficacy of praziquantel in Schistosoma haematobium-infected children in Taraba State, northeast Nigeria: a follow-up study. Sultan Qaboos Univ Med J. 2018;18(3):e304-e310. doi: 10.18295/squmj.2018.18.03.007. Available from: https://journals.squ.edu.om/index.php/squmj/article/view/304.
- N’Goran EK, Gnaka HN, Tanner M, Utzinger J. Efficacy and side-effects of two praziquantel treatments against Schistosoma haematobium infection, among schoolchildren from Côte d’Ivoire. Ann Trop Med Parasitol. 2003;97(1):37-51. doi: 10.1179/000349803125002553. Available from: https://www.tandfonline.com/doi/abs/10.1179/000349803125002553.
- Senghor B, Mathieu-Begné E, Rey O, Doucouré S, Sow D, Diop B, Sène M, Boissier J, Sokhna C. Urogenital schistosomiasis in three different water access in the Senegal river basin: prevalence and monitoring praziquantel efficacy and re-infection levels. BMC Infect Dis. 2022;22:968. doi: 10.1186/s12879-022-07813-5. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-022-07813-5.
- Picquet M, Vercruysse J, Shaw DJ, Diop M, Ly A. Efficacy of praziquantel against Schistosoma mansoni in northern Senegal. Trans R Soc Trop Med Hyg. 1998;92(1):90-93. doi: 10.1016/S0035-9203(98)90971-3. Available from: https://academic.oup.com/trstmh/article/92/1/90/1862346.
- Webster BL, Diaw OT, Seye MM, Faye DS, Stothard JR, Sousa-Figueiredo JC, Rollinson D. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: monitoring treatment success and re-infection patterns. Acta Trop. 2013;128(2):292-302. doi: 10.1016/j.actatropica.2012.09.010. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0001706X12003154.
- Augusto G, Magnussen P, Kristensen TK, Appleton CC, Vennervald BJ. The influence of transmission season on parasitological cure rates and intensity of infection after praziquantel treatment of Schistosoma haematobium-infected schoolchildren in Mozambique. Parasitology. 2009;136(13):1771-1779. Available from: https://www.cambridge.org/core/journals/parasitology/article/abs/influence-of-transmission-season-on-parasitological-cure-rates-and-intensity-of-infection-after-praziquantel-treatment-of-schistosoma-haematobiuminfected-schoolchildren-in-mozambique/….
- Webster BL, Culverwell CL diversification within Schistosoma haematobium on Zanzibar reveals substantial genetic diversity and two major phylogenetic groups. Acta Trop. 2013;128(2):206-217. doi: 10.1016/j.actatropica.2012.06.002. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0001706X12002288.
- Kahama AI, Odek AE, Kihara RW, Vennervald BJ, Kombe Y, Nkulila T, Hatz CF, Ouma JH, Deelder AM. Urine circulating soluble egg antigen in relation to egg counts, hematuria, and urinary tract pathology before and after treatment in children infected with Schistosoma haematobium in Kenya. Am J Trop Med Hyg. 1999;61(2):215-219. doi: 10.4269/ajtmh.1999.61.215. Available from: https://www.ajtmh.org/view/journals/tpmd/61/2/article-p215.xml.
- Bergquist NR, Colley DG. Schistosomiasis vaccine: research to development. Parasitol Today. 1998;14(3):99-104. doi: 10.1016/S0169-4758(97)01207-6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0169475897012076.
- Boon NAM, Van Den Broeck F, Faye D, Volckaert FAM, Mboup S, Polman K, Huyse T. Barcoding hybrids: heterogeneous distribution of Schistosoma haematobium × Schistosoma bovis hybrids across the Senegal River Basin. Parasitology. 2018;145(5):634-645. doi: 10.1017/S0031182018000525. Available from: https://www.cambridge.org/core/journals/parasitology/article/abs/barcoding-hybrids-heterogeneous-distribution-of-schistosoma-haematobium-schistosoma-bovis-hybrids-across-the-senegal-river-basin/….
- Leger E, Webster JP. Hybridizations within the genus Schistosoma: implications for evolution, epidemiology and control. Parasitology. 2017;144(1):65-80. doi: 10.1017/S0031182016001190. Available from: https://www.cambridge.org/core/journals/parasitology/article/abs/hybridizations-within-the-genus-schistosoma-implications-for-evolution-epidemiology-and-control/….
- Norton AJ, Gower CM, Lamberton PHL, Webster BL, Lwambo NJS, Blair L, Fenwick A, Webster JP. Genetic consequences of mass human chemotherapy for Schistosoma mansoni: population structure pre- and post-praziquantel treatment in Tanzania. Am J Trop Med Hyg. 2010;83(4):951-957. Available from: https://www.ajtmh.org/view/journals/tpmd/83/4/article-p951.xml.
- Bergquist R, Utzinger J, Keiser J. Controlling schistosomiasis with praziquantel: how much longer without a viable alternative? Infect Dis Poverty. 2017;6:74. doi: 10.1186/s40249-017-0286-2. Available from: https://idpjournal.biomedcentral.com/articles/10.1186/s40249-017-0286-2.
- De Moira AP, Fulford AJC, Kabatereine NB, Kazibwe F, Ouma JH, Dunne DW, Booth M. Microgeographical and tribal variations in water contact and Schistosoma mansoni exposure within a Ugandan fishing community. Trop Med Int Health. 2007;12(6):724-735. doi: 10.1111/j.1365-3156.2007.01842.x. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1365-3156.2007.01842.x.
