Research | Open Access | Volume 8 (3): Article 57 | Published: 28 Jul 2025
Implementing a rapid cascade training model to enhance laboratory response during the Sudan virus disease outbreak in Uganda, 2022
Menu, Tables and Figures
On Pubmed
- Roggers Michael Eilu
- Morgan Otita
- Daniel Kiiza
- Benson Musinguzi
- Gerald Aluma
- Ronald Ocatre
- Claire Nankoma
- Andrew Kwiringira
- Paddy Mutungi Tukamuhebwa
- Dathan Byonanebye
- Maureen Kesande
- Boneventure Brian Kawere
- Ibrahim Mugerwa
- Ritah Namusoosa
- Atek Kagirita
- Thomas Nsibambi
- Judith Nanyondo
- Amy Boore
- Suzan Nabadda
- Francis Kakooza
- Mohammed Lamorde
On Google Scholar
- Roggers Michael Eilu
- Morgan Otita
- Daniel Kiiza
- Benson Musinguzi
- Gerald Aluma
- Ronald Ocatre
- Claire Nankoma
- Andrew Kwiringira
- Paddy Mutungi Tukamuhebwa
- Dathan Byonanebye
- Maureen Kesande
- Boneventure Brian Kawere
- Ibrahim Mugerwa
- Ritah Namusoosa
- Atek Kagirita
- Thomas Nsibambi
- Judith Nanyondo
- Amy Boore
- Suzan Nabadda
- Francis Kakooza
- Mohammed Lamorde
Navigate this article
Tables
| Key area | Key competence or skill |
|---|---|
| EVD overview and the role of the laboratory in outbreak management. | – Understand the epidemiology, pathogenicity, transmission, and confirmation of EVD. – Key roles played by the laboratory responders. |
| EVD quality management systems and the national testing algorithm. | – Quality management systems in the context of the EVD response – Implementation of pre-examination, examination, and post examination procedures – Development and enforcement of SOPs. |
| Sample types, safe sample handling, and diagnostics | – Sample collection from different patient categories such as suspected cases, confirmed cases, cadavers, survivors – Ensuring safety during collection, packaging, and referral of samples – Testing technologies, and testing algorithm – Completion of Case Investigation Forms (CIFs) |
| Biosafety and biosecurity considerations | – Biosafety and biosecurity principles for personnel safety – Universal safety precautions in handling high risk pathogens – Biosecurity of samples and isolated organisms and information security of patient results |
| Infection Prevention and Control (IPC) in relation to EVD | – Standard contact precautions and practices – Practical sessions on preparation of 0.05%, 0.5% bleach solution – Donning and doffing of PPE – Waste management |
| Sample referral and results management | – Enhancements of the national hub sample transportation and results system to facilitate real-time referral and results return |
| Logistics management overview | – Requisition, stock management, and stock re-distribution, appreciate the types of supplies required and the sources available in the country |
Table 1: Competencies included in the EVD laboratory rapid response training package
| National Referral/ Specialized Hospital | Number Trained |
|---|---|
| Mulago National Referral Hospital | 34 |
| Butabika National Referral Hospital | 10 |
| Kiruddu National Referral Hospital | 16 |
| Kawempe National Referral Hospital | 5 |
| Uganda Heart Institute | 3 |
| Uganda Cancer Institute | 9 |
| Mulago Specialized Women’s National Hospital | 7 |
| Naguru Regional Referral Hospital | 5 |
| Total | 89 |
Table 2: National TOTs for the Rapid cascade training model to improve the capacity of Laboratory response teams, by health facility level, Uganda 2022
| Region | Regional training (n) | District training (n) | Sample transporters (n) | Mortuary attendants (n) | Total |
|---|---|---|---|---|---|
| Masaka | 37 | 209 | 24 | 13 | 283 |
| Jinja | 38 | 195 | 21 | 17 | 271 |
| KMA | 25 | 60 | 7 | 50 | 142 |
| West Nile | 33 | 221 | 18 | 16 | 286 |
| Total | 133 | 685 | 70 | 96 | 982 |
Table 3: Participants at the Regional and district levels for the Rapid Cascade training model to improve the capacity of Laboratory response teams, by region, Uganda 2022
| Pre-test Mean (SD) | Post-test Mean (SD) | Mean Difference | P-value | |
|---|---|---|---|---|
| Level of healthcare | ||||
| National | 67.7 (11.4) | 91.9 (7.9) | 24.2 | <0.01 |
| Regional | 62.7 (12.1) | 89.2 (10.2) | 26.5 | <0.001 |
| District | 61.1 (15.0) | 90.9 (10.5) | 29.8 | <0.001 |
| Laboratory teams | ||||
| DLFP | 61.4 (12.1) | 89.3 (9.4) | 27.9 | <0.001 |
| General Lab staff | 62.0 (14.6) | 91.4 (9.8) | 29.4 | <0.001 |
| Hub coordinators | 61.4 (11.1) | 91.4 (7.9) | 30.0 | <0.001 |
| Mortuary Attendant | 36.9 (13.5) | 65.1 (13.8) | 28.2 | <0.001 |
Table 4: Knowledge scores on EVD before and after the cascaded training
Figures




Keywords
- Rapid cascade training
- Laboratory response teams
- Sudan virus disease
Roggers Michael Eilu1, Morgan Otita1, Daniel Kiiza1, Benson Musinguzi2,3, Gerald Aluma1, Ronald Ocatre1, Claire Nankoma2, Andrew Kwiringira1, Paddy Mutungi Tukamuhebwa1, Dathan Byonanebye1, Maureen Kesande1, Boneventure Brian Kawere1, Ibrahim Mugerwa2, Ritah Namusoosa2, Atek Kagirita2, Thomas Nsibambi4, Judith Nanyondo1, Amy Boore4, Suzan Nabadda2, Francis Kakooza1, Mohammed Lamorde1
1Department of Global Health Security, Infectious Diseases Institute, Makerere University, Kampala, Uganda, 2Department of National Health Laboratory and Diagnostic Services, Ministry of Health, Kampala, Uganda, 3Department of Medical Laboratory Science, Faculty of Health Sciences, Muni University, Arua, Uganda, 4United States Centres for Disease Control and Prevention.
&Corresponding author: Roggers Michael Eilu, Department of Global Health Security, Infectious Diseases Institute, Kampala, Uganda, Email: reilu@idi.co.ug, ORCID: https://orcid.org/0009-0006-8113-4562
Received: 28 Apr 2025, Accepted: 25 Jul 2025, Published: 28 Jul 2025
Domain: Outbreak Investigation, Field Epidemiology
Keywords: Rapid cascade training, Laboratory response teams, Sudan virus disease
©Roggers Michael Eilu et al Journal of Interventional Epidemiology and Public Health (ISSN: 2664-2824). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Roggers Michael Eilu et al Implementing a rapid cascade training model to enhance laboratory response during the Sudan virus disease outbreak in Uganda, 2022. Journal of Interventional Epidemiology and Public Health. 2025;8(3):57. https://doi.org/10.37432/jieph-d-25-00105
Abstract
Introduction: In September 2022, Uganda experienced an outbreak of Sudan Virus Disease. We describe the roll-out of a rapid cascade training model which was implemented to enhance the capacity of laboratory response teams in managing samples at both national and subnational levels.
Method: A training package was developed, reviewed by laboratory pillar members and approved by the Ministry of Health. The laboratory pillar team facilitated a national training program for trainers from national and specialized referral laboratories. Officers trained at the national level then trained regional laboratory officers, who trained district-level trainers and personnel from health facilities. The mean pre-test and post-test scores were compared using the paired t-test to evaluate knowledge improvement among participants. The turnaround time for laboratory results before and after training was monitored using the national results dispatch system.
Results: Within two weeks of the development of the training package, 89 national and 133 regional trainers were trained, alongside 685 district laboratory response teams, 60 hub riders and drivers, and 86 mortuary attendants. There was a marked improvement in post-test knowledge assessments versus pre-test scores at both national and sub-national levels, as well as across all targeted health worker cadres from 59% to 87% (p<0.001). The swift increase in the number of personnel available to collect and refer samples post-training contributed to a rise in the number of samples collected, from 315 in the initial two weeks of response prior to the training, to a cumulative total of 4,430 by the conclusion of the outbreak. Additionally, the turnaround time was reduced from 144 hours before training to 24 hours after training.
Conclusion: Implementing rapid cascade training during an outbreak response is both practical and advantageous. It boosts laboratory personnel’s confidence in handling high-risk pathogen samples and enhances sample quality. The quick increase in trained laboratory staff in the intervention areas led to more personnel available for quality sample management, reduced fatigue among the initially small team, and consequently, a shorter turnaround time.
Introduction
Ebola virus disease (EVD) continues to pose a significant public health challenge in sub-Saharan Africa [1]. The case fatality rate due to EVD is notably high, ranging from 25% to 90%, contingent upon the viral strain and the quality of supportive care administered to the patient [2]. Effective containment necessitates the involvement of skilled epidemiological, clinical, and laboratory personnel to ensure a comprehensive response. In September 2022, the Ugandan Ministry of Health (MOH) reported an outbreak of the Sudan strain of EVD [3], marking the seventh such outbreak in Uganda [4]. During this outbreak, Uganda reported 164 cases, with 55 confirmed fatalities and 87 individuals recovering from the illness [3].
A proficient laboratory workforce is essential for confirming disease etiologies and executing diagnostic tests for proper clinical case management [5]. During the 2022 outbreak, collecting samples from suspected cases while ensuring laboratory personnel’s safety was challenging. Reports from laboratory meetings showed staff were hesitant to collect samples due to perceived risk, particularly in high-risk regions [6]. Due to limited EVD knowledge, sample collectors used inappropriate Personal Protective Equipment (PPE) like long-sleeved gowns and surgical masks, instead of N95 respirators or Powered Air Purifying Respirators. They collected insufficient samples, used incorrect blood collection tubes, failed to follow triple packaging guidelines, and/or collected inappropriate samples [6]. Sample transporters in the national transport network, fearing infection, placed samples in two layers of biohazard bags, delaying delivery to reference laboratories in the first two response weeks [6]. Lab pillar updates indicated viral hemorrhagic fever (VHF) case investigation forms lacked critical information, and turnaround time (TAT) from collection to results availability exceeded 72 hours due to collection and transport delays [6].
To address these concerns, it was imperative that laboratory responders receive comprehensive training in safe sample management, biosafety, and biosecurity, particularly in the context of handling high-risk pathogens, as the risk of exposure is significantly elevated for untrained individuals [7, 8]. Rapid training models are increasingly advocated for as an effective means of delivering precise information [9], instructions, and guidelines in situations where they are urgently needed but often cannot be completed in a timely manner. Cascade training is an approach designed to effectively train a large number of individuals within programs or institutions. This method starts by training a group of master trainers, who then go on to train smaller groups, continuing this process until all required personnel have received training [10]. This paper elucidates the implementation of a rapid cascade training model, a standardized stepwise approach designed to facilitate swift, structured training at national, regional, district, and facility levels, thereby reaching a large number of responders across a broad geographical area. The rapid cascade training model sought to enhance the capacity of laboratory response teams in managing samples at the national, regional, and district levels during outbreak responses. We detail the deployment of this rapid cascade training model for the laboratory workforce in four high-risk health regions in Uganda, aimed at strengthening the capacity and skills of the laboratory workforce to effectively respond to the 2022 Ebola-SVD outbreak in Uganda.
Methods
Geographical scope and setting
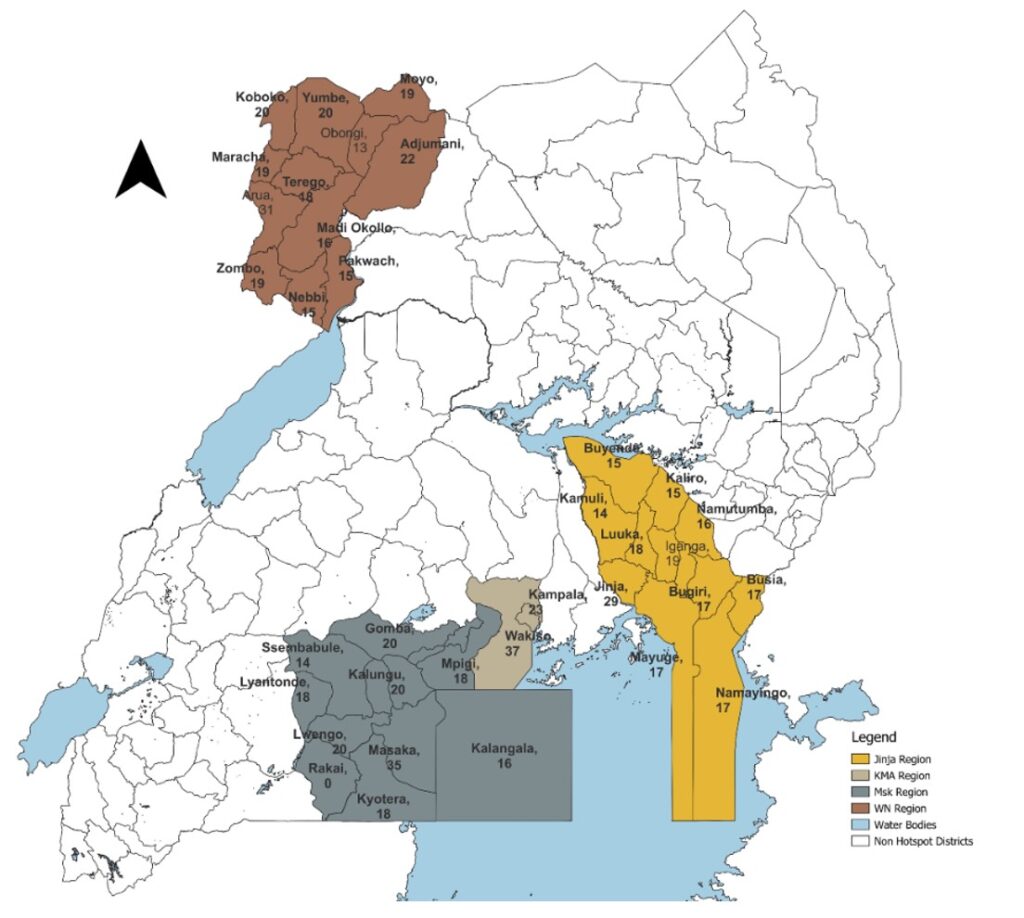
The four high-risk regions, namely Arua, Jinja, Masaka and Kampala (Figure 1), were selected based on the identification of at least one confirmed case in each area. Additionally, the Arua Region was deemed high risk due to significant mobility across the porous borders with Uganda, the Democratic Republic of the Congo (DRC), and South Sudan. All districts within these selected regions received support to enhance their laboratory capacity through this training model.
The criteria for selecting high-risk health facilities to prioritize for the training included the occurrence of at least one EVD case, or proximity to an incident case, or a high level health facility (level four (IV) and above), patient number proximity to a point of entry or border, the facility’s potential to host isolation or holding units, linkage to the hub system, and the presence of laboratory personnel.
Participants for the training were selected based on the level of care of the health facilities they work at, laboratory technical staff at the various levels of care as per facilities identified. The other special teams included sample transporters and morticians or mortuary attendants who assisted in sample collection from dead bodies.
Implementation of rapid cascade training model
Development of National Reference materials for the Ebola Laboratory Training
The laboratory training subcommittee, comprising representatives from the Ministry of Health (MOH) and health partners forming the training technical working group (TWG) of the National Laboratory pillar, conducted a comprehensive desk review of existing national laboratory-based training materials. The documents reviewed included the WHO-EVD preparedness training package [11], Standard Operating Procedures (SOPs) and Guidelines for Responding to Ebola/Marburg virus Disease Outbreaks in Uganda [12], and selected publications such as the “WHO Ebola and Marburg Virus Disease Epidemics: Preparedness, Alert, Control and Evaluation” [13] as well as a comprehensive review of EVD by Jaco et al [1]. These training materials were specifically developed to address Uganda’s needs and to fill an information and competency gap regarding personal protective equipment and procedures necessary for Ebola sample collection, sample type, storage conditions for maintaining sample integrity, sample referral and reference testing, and logistics for sample management, as observed in the early phase of the response (Table 1).
Additionally, the team developed video-based training materials that laboratory responders could download and view at their convenience. The developed training materials were subsequently presented to the laboratory pillar for review and approval. The approved Ebola National Reference Training materials were employed to conduct training at all levels using a rapid and cascading model. The training was structured as a two-day program for training of trainers (TOT) and a one-day program for district-level teams. A training program, an evaluation tool, and pre-test and post-test tools (Annex 1) were developed to assess the knowledge gained by participants. A passing mark was established at 80% for TOTs, in accordance with the College of American Pathologists proficiency testing/External Quality Assessment (EQA) satisfactory score [14], and 60% for other laboratory response teams, in alignment with national training norms.
Cascade training model
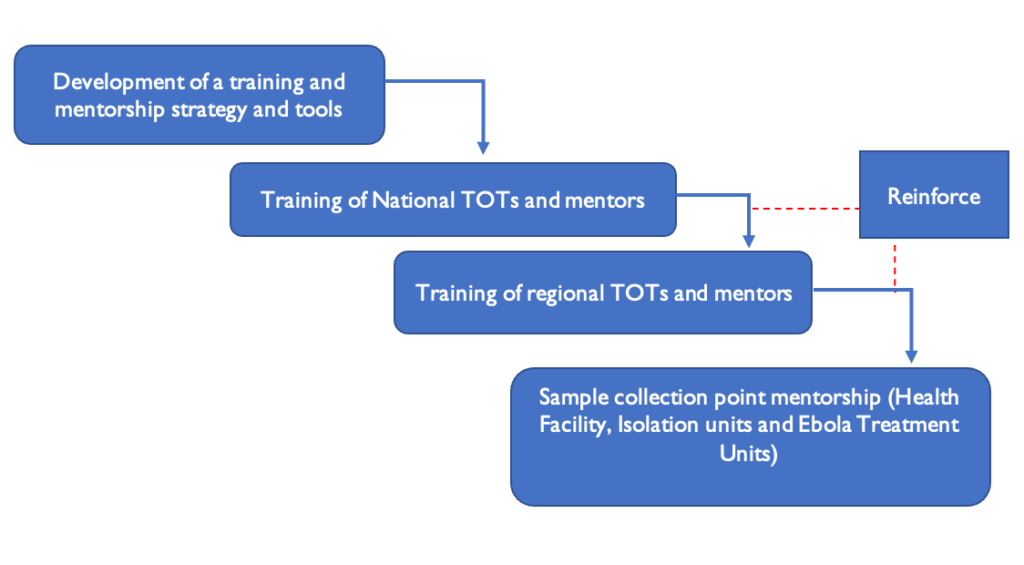
The training initiative focused on national and regional trainers, as well as district laboratory response teams. The national trainers from the laboratory pillar training subcommittee carried out the national Training of Trainers (TOT), and these national trainers then held regional TOT sessions under the guidance of the subcommittee trainers. A national laboratory trainer accompanied the regional trainer to support and mentor the district laboratory trainers/mentors, or alternatively, a regional trainer worked alongside district laboratory trainers to provide training and mentorship at the facility/sample collection points, serving as a quality assurance measure for the training and mentorship (Figure 2).
National trainers were selected based on their laboratory expertise and prior experience with previously supported outbreaks or their involvement in the national laboratory pillar. These trainers were tasked with training national Trainers of Trainers (TOTs). Participants for the national TOT were selected from the national referral hospital, regional referral hospitals, and implementing partners. The regional TOTs were selected from regional referral hospitals, districts, health sub-districts, and regional implementing partners. District laboratory response officers were sourced from district hospitals, and high-volume health facilities at levels four (HCIV) and three (HCIII). A total of 15 individuals from the district were chosen for the training, who would subsequently become part of the district response teams.
Training approaches
To facilitate rapid expansion, extensive reach, and effective knowledge acquisition by laboratory responders regarding safe sample management, two training methodologies were implemented in a phased approach. The initial method involved virtual orientations and the dissemination of video materials through Zoom sessions and social media platforms, such as WhatsApp, among laboratory network groups. The laboratory pillar administrator organized one-hour Zoom sessions, accommodating up to 300 participants, and encouraged participation through phone calls and emails. The second method comprised in-person training sessions. Government officials were responsible for on-site training, and the Director General of Health issued letters to the District Health Officer (DHO), Chief Administrative Officer (CAO), and district health authorities, requesting their participation and the provision of a venue for the training sessions. These sessions incorporated didactic instruction, demonstrations, playback of instructional videos, and practical activities focused on identifying personal protective equipment (PPE), donning and doffing procedures, waste segregation and disposal, disinfectant preparation, sample collection from both mobile and immobile patients, and sample collection from cadavers, specifically for mortuary attendants. The practical sessions were conducted at the district hospital mortuary.
The participants knowledge was assessed using a standardized pre- and post-test form. A two-day National TOT was held, and under the supervision of the laboratory pillar trainers, the participants underwent teach-back sessions where trainees were tasked to deliver a session to the participants and were evaluated for teaching and knowledge dispensation ability. An acceptable post-test score of >80% was utilized to declare candidates as national trainers. The national TOTs were further evaluated on knowledge and teaching methods using an internal checklist for trainers. Under the supervision of national laboratory pillar trainers, national TOTs led a two-day training for regional trainers. A candidate was considered a competent regional trainer if they received an acceptable post-test score of over 80%. The regional TOTs were evaluated on their knowledge and competency. Under the supervision of the national laboratory pillar trainers, the national and regional TOTs led a one-day training for district laboratory rapid response teams. The district laboratory response teams’ knowledge and competency were evaluated, and a passing post-test score above 60% was utilised to determine the participants’ competence.
Data collection and analysis
Data regarding turnaround times before and after the intervention were sourced from the national electronic results dispatch system, which records the date and time of sample collection, reception, and results dispatch. Details about the number, types, and locations of health facilities, districts, and regions involved in the training sessions, along with their pre- and post-test scores, were extracted from training reports. The paired t-test was employed to analyse the differences in mean scores before and after the training sessions. Likewise, the paired t-test was used to assess the mean turnaround times of samples collected prior to and following the cascaded training sessions. Assumptions of the paired t-test were tested by assessing for normality of the differences between pre- and post-test scores graphically using a histogram, as well as the Shapiro-Wilk test and the differences were normally distributed. Bar charts were used to depict sample volumes and the variations in turnaround times before and after the intervention.
Ethical approval
Approval for this project was obtained from the Ministry of Health (MOH), and administrative approval was sought from District Health Officers. The research office at the Infectious Disease Institute determined that the primary objective of this evaluation was to engage in public health practice, and therefore, it was not categorized as human subject research. To ensure participant confidentiality during data collection, unique identifiers were employed in lieu of names. The data was stored on password-protected computers and was not disclosed to individuals outside the project team. Informed consent was not obtained from the participants because this intervention was conducted as part of a routine capacity-building effort in the context of a public health response to the EVD outbreak. The training did not involve any experimental procedures, collection of sensitive personal data, or exposure to risk more than what participants would normally encounter in their day-to-day professional duties.
Results
During the third week of the Ebola Virus Disease (EVD) response, training sessions were organised for healthcare workers in four high-risk regions, covering 41 out of 146 districts in Uganda. These regions included five divisions and two districts of the Kampala Metropolitan Area (KMA), 13 districts in the Masaka Region, 13 in the Jinja Region, and 13 in the Arua Region. Using the Ministry of Health (MOH) approved EVD national training materials, four national virtual training sessions were conducted. The rapid cascade training commenced with a two-day session for 89 laboratory staff from national referral and specialized hospitals, who were trained as National Trainers of Trainers (TOTs) (Table 2). At the regional level, 133 laboratory staff were trained as regional trainers across the four high-risk regions over six days, with two days allocated per region. Over eight days, the team trained 70 sample transporters as hub riders and 96 mortuary attendants, dedicating one day per district. The mortuary attendants were trained to enhance sample collection from cadavers at mortuaries and within communities. The district-level training involved 685 participants (Table 3).
Change in knowledge
There was a significant gain in knowledge across all cadres trained and across all levels of care (Table 4) with an overall improvement in knowledge from 59% at baseline to 87% after the training (p<0.001)
Outcomes of the training
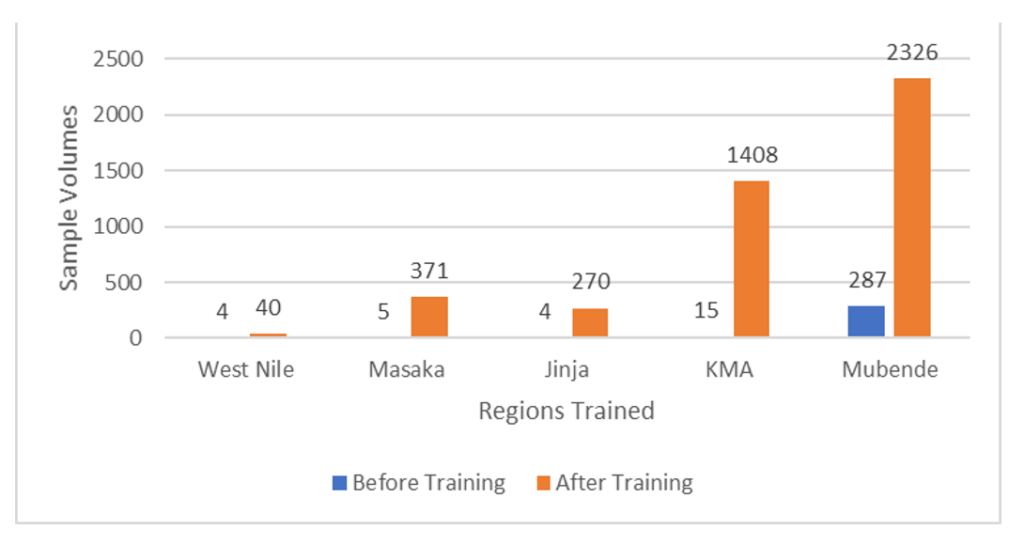
Following a 15-day intensive cascade training program, a total of 1,071 proficient laboratory personnel were mobilized to facilitate sample collection. These trained individuals were instrumental in the collection of 93% (4415/4,730) of the samples, which were subsequently transported to reference laboratories for analysis over a 69-day response period. Before the training, the limited number of laboratory responders and the cases reported at that time resulted in the collection of only 315 samples. Post-training, there was a significant increase of 93% in the number of samples collected, totalling 4,415, as the number of suspected cases escalated during the outbreak. Out of 4,415 samples collected, 52.5% (2326/4415) originated from Mubende District, 31.8% (1408/4415) from KMA, 8.4% (371/4415) from Masaka District, 6% (270/4415) from Jinja District, and 1% (40/4415) from West Nile (Figure 3). Mubende Region was the original source of the outbreak and contributed most of the samples for suspected cases. However, the rapid cascade training was to restricted to the four regions where the outbreak was spreading to.
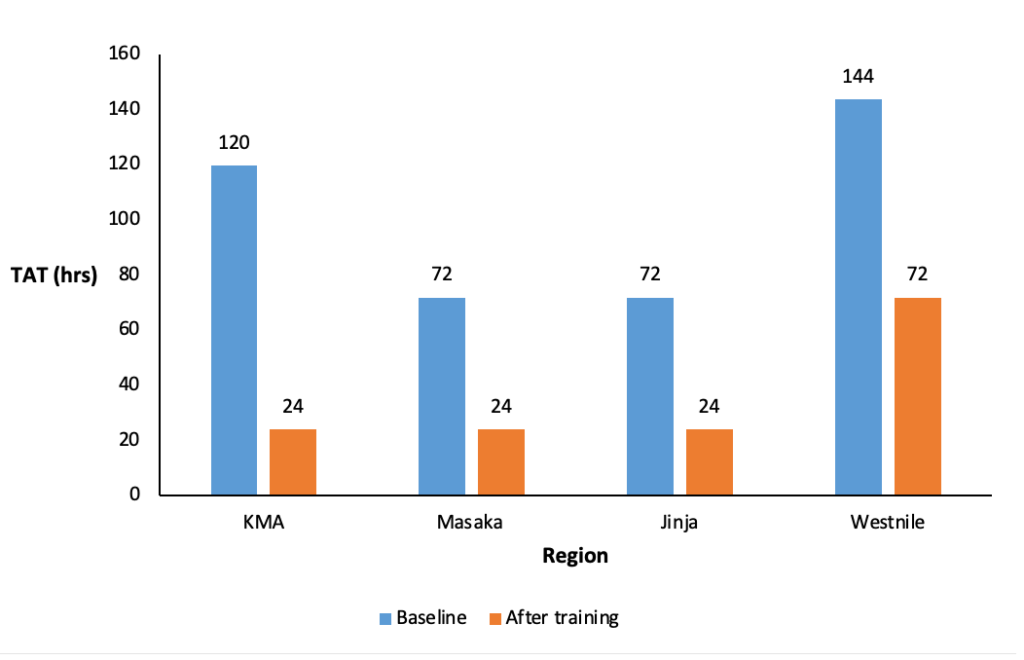
Turnaround time (TAT) reduced from 144 hours before the training cascade (baseline) to 24 hours after cascade training in West Nile, 120 hours to 24 hours in KMA, 72 hours to 24 hours in Masaka region and 72 hours to 24 hours in Jinja region (Figure 4).
Implementation challenges and opportunities
Dealing with a highly dangerous pathogen that posed significant risks and for which no treatment or vaccine was available made it exceptionally challenging to recruit staff for sample collection unless specific and well-defined roles with appropriate compensation were established. The majority of staff who took part in the training sessions were mobilized by the DLFPs at the direction of the DHO.
The rapid spread of the pathogen within Mubende and to various other regions, including KMA, Masaka, and Jinja, necessitated the swift deployment of teams to the affected regions. However, this was contingent on logistical factors like the movement orders of trainers and resource mobilisation for training necessities such as accommodation and training venues. The Ministry of Health, National Health Laboratory and Diagnostics Services Department and the national laboratory pillar of the Ebola incident management team, provided leadership and coordination during these efforts. Funding for associated training costs was provided by the Infectious Diseases Institute with co-funding from US Centers for Disease Control and Prevention (CDC) and World Health Organization (WHO).
A pre-existing laboratory network structure was already in place, with staff available at the district, regional, and national levels. Private institutions also permitted their laboratory personnel to participate in the training, particularly in response to requests from the district health officers. These officers not only offered training venues but also played a crucial role in mobilizing participants for the training sessions, contributing to a seamless and effective training process.
Discussion
We present a rapid cascade training model aimed at enhancing the capacity of laboratory response teams during the 2022 Sudan Virus Disease outbreak response in Uganda. Through this cascaded training intervention, a total of 1,071 laboratory personnel were mobilized across various levels of the health system and trained to facilitate EVD sample collection. This initiative resulted in an increase in the number of samples collected, from 315 prior to the training to 4,430 samples post-training. The training sessions led to an enhancement in EVD knowledge, as evidenced by a significant improvement in mean scores from 59% to 87% before and after the training, respectively. Additionally, the mean turnaround time (TAT) for EVD results improved across all geographical regions supported by this training.
Considering the cascade training method has been extensively utilized in healthcare settings, its use during public health emergencies in Uganda has been relatively limited. A notable exception is a study by Wasukila et al., which implemented this approach to educate health workers on emergency care amid the COVID-19 pandemic in 2021, resulting in the training of 561 health workers across various care levels [15]. The research by Wasukila et al. demonstrated that the cascade training model is an effective means of disseminating emergency care knowledge to a large number of healthcare professionals in resource-limited settings, as evidenced during the COVID-19 crisis in Uganda. Their findings indicated that the cascade model enabled the training of seven times more healthcare workers than those initially trained by master trainers, underscoring its efficacy in knowledge dissemination and its potential for replication in similar contexts [15]. Similarly, our study also demonstrated that this approach contributed to an increase in the laboratory workforce skilled in safe and quality sample collection, packaging, and transportation, which led to a swift rise in the number of samples collected from patients needing sample collection, ultimately reducing the turnaround time (TAT).
Following the training, the rise in the number of laboratory samples could indicate several possibilities, such as enhanced productivity and efficiency of the laboratory staff, better coordination, and timely transportation of samples. Although there is limited research on how training affects the productivity of laboratory teams, Awang et al. have suggested that training generally enhances employees’ knowledge, skills, and positive work attitudes, ultimately leading to better job performance. Furthermore, evidence indicates that training programs can help achieve work goals and improve the quality of products or services offered [16]. However, the increase in laboratory samples might also have been influenced by other simultaneous outbreak initiatives, such as the provision of PPE and sample collection supplies. Therefore, the rise in collected laboratory samples following the rapid cascade training cannot be solely attributed to the training itself.
One of the most important indicators of laboratory service is TAT, which is frequently utilised as a key performance measure of laboratory performance [17]. Early receipt of laboratory results within 24 hours and up to a maximum of 48 hours, particularly for diseases that are epidemic-prone, facilitates early detection and control of disease outbreaks in animals and prevention of human exposure to these outbreaks [17]. There was an observed improvement in TAT after the training in all study regions; however, TAT remained above the target of 24 hours in the West Nile Region but below 48 hours. Since the West Nile Region was not at the epicentre of the outbreak, there was no mobile laboratory deployed in that region compared to other regions. West Nile region referred its samples to a reference laboratory in Kampala, about 600km away. The improvement in turnaround time in other regions can also be attributed to the availability of mobile laboratories within those regions [18].
Post-training test scores indicated a substantial increase in knowledge for all groups of trainees. These findings suggest that even in an active public health crisis, rapid training can be conducted and result in immediate improvements in knowledge and practical skills. The CDC safety training course for healthcare workers during the response to the Ebola Virus Disease also demonstrated a rapid change in knowledge following training[19]. More follow-ups would be required to know whether the trainees experienced sustained knowledge retention and reduced their risk for Ebola for a longer duration following the training. The rapid cascade training model deployed a blended learning solution to optimise efficiency and effectiveness. The model included asynchronous Web-based content, on-site live training, and an approach shown to further cognitive development. This approach allowed us to address combinations of cognitive and psychomotor skills improving knowledge, skills and confidence amongst healthcare workers. A similar result has been illustrated by a training program for Hand hygiene and biomedical waste management that was designed to improve cognitive and psychomotor domains in northern India[20].
Strong leadership, coordination, and collaboration between the Ministry of Health (at all levels), National reference laboratory network, such as Uganda Virus Research Institute (UVRI), National Health Laboratory Diagnostic Service (NHLDS), and implementing partners, enabled rapid cascade training programs. The incident management team works through pillars and mandates pillars to have command, set up structures and improve bottlenecks identified during the response period[21]. The National Laboratory pillar set up this training program and mobilised resources, including overall coordination of the training program. To sustain these gains and improve epidemic detection for surge capacity, regular supportive in-service training, supervision, simulations, and table top exercises are crucial.
The capacity built through this training could be used to combat future outbreaks of viral haemorrhagic fevers. Moreover, several key components of the training, such as outbreak management, IPC practices, and logistics management, have considerable applicability to outbreaks of other pathogens that affect resource-limited settings.
Limitations
The implementation of rapid cascade training conducted in isolation may not have directly influenced the observed increase in the number of samples collected or the reduction in TAT, as various factors contribute to these outcomes. Furthermore, the team did not conduct any follow-up assessments with the trained individuals post-outbreak to evaluate the retention of knowledge acquired during the training.
Conclusion
Implementation of a rapid emergency training during outbreak response is feasible, and it improves the confidence of laboratory staff in handling samples from high-consequence pathogens and enhances the quality of samples collected, thereby improving the quality of the results produced. The rapid swell-up of a number of trained laboratory staff within the affected regions meant more persons available to support quality sample management, less fatigue on the few that were available, and hence quality results in short TAT. Leveraging on pre-existing systems, resources, and partnerships with the availability of training resources is key in the implementation of rapid cascade trainings. Regular supportive in-service supervision, continuous medical education and training, simulations, and tabletop exercises could be implemented to maintain current competencies, preparedness, and sustainability.
What is already known about the topic
- Ebola Virus Disease is an extremely deadly filovirus infection necessitating timely and coordinated public health interventions in affected regions or those at high risk
- The capacity of laboratories to respond is vital during outbreaks for the rapid confirmation of cases, safe handling of specimens, and effective monitoring, yet this capacity is frequently lacking in many countries
- Cascade training models have proven effective during health emergencies to quickly enhance workforce capabilities by training master trainers who then provide standardized training at regional and district levels. In Uganda, this has been demonstrated through training of health workers on emergency medical services during the 2021 COVID-19 pandemic
What this study adds
- This study demonstrates the application of a rapid cascade training model, highlighting its effectiveness in quickly boosting the number of laboratory staff during an SVD outbreak in high-risk regions of Uganda
- It establishes a scalable framework for layered training, starting with national trainers and expanding to regional and district-level responders, thereby enhancing readiness for future public health emergencies
- It highlights the integration of virtual training methods with in-person components to overcome logistical and geographical challenges, particularly during emergencies. Additionally, it illustrates how targeted training for critical roles such as laboratory technicians, mortuary staff, and sample couriers led to quicker diagnostic results and improved specimen handling safety
Funding
This project was funded by the United States Centres for Disease Control and Prevention (CDC) through the Infectious Disease Institute, Makerere University. No other funding was obtained for data analysis and the development of this manuscript. The funder had no role in the design of the study, collection of data, or decision to publish the work.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the U.S. Centres for Disease Control and Prevention.
Acknowledgements
We acknowledge the MOH and IDI for the technical support during the execution of this project. We appreciate the CDC for funding the project. We also acknowledge District Health Teams and health workers for their participation. No other funding was obtained for data analysis and the development of this manuscript.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
List of abbreviations
CDC: United States Centre for Disease Control and Prevention
DHO: District Health Officer
DLFP: District Laboratory Focal Person
EQA: External Quality Assessments
EVD: Ebola Virus Disease
IPC: Infection Prevention and Control
KCCA: Kampala Capital City Authority
KMA: Kampala Metropolitan Area
MOH: Ministry of Health
NHLDS: National Health Laboratory Diagnostic Service
PPE: Personal Protective Equipment
SOP: Standard Operating Procedures
SVD: Sudan Virus Disease
TAT: Turn Around Time
UVRI: Uganda Virus Research Institute
VHF: Viral Haemorrhagic Fever
WHO: World Health Organisation
Authors´ contributions
RME, MO, DK, BM, GA, ML, RO, CN, AK, DMB, MK, BBK, IM, RN, AK, TN, JN, AB, SN, and ML contributed to the conception, design and preparation of the draft manuscript, while RME, AK, PMT, DMB and MK supported the analysis, interpretation and preparation of the results and preparation of the manuscript. All authors read through and approved the final version of the manuscript.
| Key area | Key competence or skill |
|---|---|
| EVD overview and the role of the laboratory in outbreak management. | – Understand the epidemiology, pathogenicity, transmission, and confirmation of EVD. – Key roles played by the laboratory responders. |
| EVD quality management systems and the national testing algorithm. | – Quality management systems in the context of the EVD response – Implementation of pre-examination, examination, and post examination procedures – Development and enforcement of SOPs. |
| Sample types, safe sample handling, and diagnostics | – Sample collection from different patient categories such as suspected cases, confirmed cases, cadavers, survivors – Ensuring safety during collection, packaging, and referral of samples – Testing technologies, and testing algorithm – Completion of Case Investigation Forms (CIFs) |
| Biosafety and biosecurity considerations | – Biosafety and biosecurity principles for personnel safety – Universal safety precautions in handling high risk pathogens – Biosecurity of samples and isolated organisms and information security of patient results |
| Infection Prevention and Control (IPC) in relation to EVD | – Standard contact precautions and practices – Practical sessions on preparation of 0.05%, 0.5% bleach solution – Donning and doffing of PPE – Waste management |
| Sample referral and results management | – Enhancements of the national hub sample transportation and results system to facilitate real-time referral and results return |
| Logistics management overview | – Requisition, stock management, and stock re-distribution, appreciate the types of supplies required and the sources available in the country |
| National Referral/ Specialized Hospital | Number Trained |
|---|---|
| Mulago National Referral Hospital | 34 |
| Butabika National Referral Hospital | 10 |
| Kiruddu National Referral Hospital | 16 |
| Kawempe National Referral Hospital | 5 |
| Uganda Heart Institute | 3 |
| Uganda Cancer Institute | 9 |
| Mulago Specialized Women’s National Hospital | 7 |
| Naguru Regional Referral Hospital | 5 |
| Total | 89 |
| Region | Regional training (n) | District training (n) | Sample transporters (n) | Mortuary attendants (n) | Total |
|---|---|---|---|---|---|
| Masaka | 37 | 209 | 24 | 13 | 283 |
| Jinja | 38 | 195 | 21 | 17 | 271 |
| KMA | 25 | 60 | 7 | 50 | 142 |
| West Nile | 33 | 221 | 18 | 16 | 286 |
| Total | 133 | 685 | 70 | 96 | 982 |
| Pre-test Mean (SD) | Post-test Mean (SD) | Mean Difference | P-value | |
|---|---|---|---|---|
| Level of healthcare | ||||
| National | 67.7 (11.4) | 91.9 (7.9) | 24.2 | <0.01 |
| Regional | 62.7 (12.1) | 89.2 (10.2) | 26.5 | <0.001 |
| District | 61.1 (15.0) | 90.9 (10.5) | 29.8 | <0.001 |
| Laboratory teams | ||||
| DLFP | 61.4 (12.1) | 89.3 (9.4) | 27.9 | <0.001 |
| General Lab staff | 62.0 (14.6) | 91.4 (9.8) | 29.4 | <0.001 |
| Hub coordinators | 61.4 (11.1) | 91.4 (7.9) | 30.0 | <0.001 |
| Mortuary Attendant | 36.9 (13.5) | 65.1 (13.8) | 28.2 | <0.001 |




References
- Jacob ST, Crozier I, Fischer WA, Hewlett A, Kraft CS, de la Vega MA, Soka MJ, Wahl V, Griffiths A, Bollinger L, Kuhn JH. Ebola virus disease. Nat Rev Dis Primers. 2020;6(1):13. Available from: https://www.nature.com/articles/s41572-020-0147-3. doi:10.1038/s41572-020-0147-3.
- Kabami Z, Ario AR, Harris JR, Ninsiima M, Ahirirwe SR, Ocero JR, Atwine D, Mwebesa HG, Kyabayinze DJ, Muruta AN, et al. Ebola disease outbreak caused by the Sudan virus in Uganda, 2022: a descriptive epidemiological study. Lancet Glob Health. 2024;12(10):e1684-92. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2214109X24002602. doi:10.1016/S2214-109X(24)00260-2.
- World Health Organization (Regional Office for Africa). Uganda declares Ebola Virus Disease outbreak [Internet]. Brazzaville: World Health Organization (Regional Office for Africa); 2022 Sep 20 [cited 2025 Jul 28]. Available from: https://www.afro.who.int/countries/uganda/news/uganda-declares-ebola-virus-disease-outbreak.
- Rai A, Hamiidah N, Abbass M, Masunga DS, Shoumar H, Kantawala BI, Wellington J, Uwishema O. Ebola virus disease in Uganda: a global emergency call. Ann Med Surg (Lond). 2022;84:104825. Available from: https://journals.lww.com/10.1016/j.amsu.2022.104825. doi:10.1016/j.amsu.2022.104825.
- Edgar M, Selvaraj SA, Lee KE, Caraballo-Arias Y, Harrell M, Rodriguez-Morales AJ. Healthcare workers, epidemic biological risks – recommendations based on the experience with COVID-19 and Ebolavirus. Infez Med. 2022;30(2):162-77. Available from: https://www.infezmed.it/media/journal/Vol_30_2_2022_2.pdf. doi:10.53854/liim-3002-2.
- National Health Laboratory and Diagnostics Services (Uganda). Laboratory subcommittee updates [oral presentation]. LAB Sub Committee Meeting; 2022 Sep 26; Kampala (Uganda).
- Sealy TK, Erickson BR, Taboy CH, Ströher U, Towner JS, Andrews SE, Rose LE, Weirich E, Lowe L, Klena JD, et al. Laboratory response to Ebola – West Africa and United States. MMWR Suppl. 2016;65(3):44-9. Available from: http://www.cdc.gov/mmwr/volumes/65/su/su6503a7.htm. doi:10.15585/mmwr.su6503a7.
- Gibbs EP. Emerging zoonotic epidemics in the interconnected global community. Vet Rec. 2005;157(22):673-9.
- Tsiouris F, Hartsough K, Poimboeuf M, Raether C, Farahani M, Ferreira T, Kamanzi C, Maria J, Nshimirimana M, Mwanza J, et al. Rapid scale-up of COVID-19 training for frontline health workers in 11 African countries. Hum Resour Health. 2022;20(1):43. Available from: https://human-resources-health.biomedcentral.com/articles/10.1186/s12960-022-00739-8. doi:10.1186/s12960-022-00739-8.
- Linked Immunization Action Network. Training best practices: cascade training [Internet]. Linked Immunization Action Network; 2019 May 20 [cited 2025 Jul 28]. Available from: https://www.linkedimmunisation.org/wp-content/uploads/2021/01/4_BestPractices_Cascade-Training.pdf.
- World Health Organization (Regional Office for Africa). EVD preparedness training package (WHO Regional Office for Africa – version 1) [Internet]. Brazzaville: World Health Organization (Regional Office for Africa); 2015 Nov [cited 2025 Jul 28]. Available from: https://extranet.who.int/hslp/content/evd-training-package-1.
- Ministry of Health (Uganda). Standard operating procedures and guidelines for responding to Ebola/Marburg Virus Disease outbreaks in Uganda [Internet]. Kampala: Ministry of Health; 2025 [cited 2025 Jul 28]. Available from: https://www.health.go.ug/wp-content/uploads/2022/09/SOP-EBOLA-MARBURG-FINAL.pdf.
- World Health Organization. Ebola and Marburg virus disease epidemics: preparedness, alert, control and evaluation [Internet]. Geneva: World Health Organization; 2024 Aug 6 [cited 2025 Jul 28]. Available from: https://iris.who.int/bitstream/handle/10665/130160/WHO_HSE_PED_CED_2014.05_eng.pdf?sequence=1.
- College of American Pathologists. Proficiency testing/external quality assessment frequently asked questions [Internet]. Washington (DC): College of American Pathologists; 2025 [cited 2025 Jul 28]. Available from: https://www.cap.org/laboratory-improvement/proficiency-testing/proficiency-testing-pt-external-quality-assessment-eqa-faq.
- Wasukira SB, Kambugu CT, Nanyondo SJ, Candia E, Aporu SE, Ikwaru P, Kwagala R, Kwiringira A, Mukiibi P, Murungi C, et al. Implementation of a cascade training model to enhance emergency care capacity of healthcare workers during the COVID-19 outbreak in Uganda. Afr J Emerg Med. 2025;15(1):565-70. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2211419X25000011. doi:10.1016/j.afjem.2025.01.001.
- Awang AH, Ismail R, Mohd Noor Z. Training impact on employee’s job performance: a self-evaluation. Econ Res. 2010;23(4):78-90. Available from: http://www.tandfonline.com/doi/full/10.1080/1331677X.2010.11517434. doi:10.1080/1331677X.2010.11517434.
- Hawkins RC. Laboratory turnaround time. Clin Biochem Rev. 2007;28(4):179-94. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC2282400/.
- Gehre F, Lagu H, Achol E, Omari N, Ochido G, Duraffour S, Hinzmann J, Kezakarayagwa E, Kabatesi F, Nkeshimana A, et al. The East African Community’s mobile laboratory network’s rapid response during the first 2 weeks of the Ebola Sudan virus disease (SVD) outbreak in Uganda and pandemic preparedness activities in South Sudan, Rwanda, Tanzania, Burundi, Kenya. BMJ Glob Health. 2022;7(12):e011073. Available from: https://gh.bmj.com/lookup/doi/10.1136/bmjgh-2022-011073. doi:10.1136/bmjgh-2022-011073.
- Narra R, Sobel J, Piper C, Gould D, Bhadelia N, Dott M, Fiore A, Fischer WA, Frawley MJ, Griffin PM, et al. CDC safety training course for Ebola virus disease healthcare workers. Emerg Infect Dis. 2017;23(13):S217-24. Available from: http://wwwnc.cdc.gov/eid/article/23/13/17-0549_article.htm. doi:10.3201/eid2313.170549.
- Rohilla R, Gupta PK, Narula H, Sharma AK, Mehta V, Rao S, Gupta J, Gupta P. Assessment of cognitive and psychomotor domains regarding biomedical waste management and hand hygiene among various categories of health-care professionals at a tertiary care center in Northern India. J Educ Health Promot. 2021;10:186. Available from: https://journals.lww.com/10.4103/jehp.jehp_884_20. doi:10.4103/jehp.jehp_884_20.
- Aceng JR, Ario AR, Muruta AN, Makumbi I, Nanyunja M, Komakech I, Bakainaga AN, Talisuna AO, Mwesigye C, Mpairwe AM, et al. Uganda’s experience in Ebola virus disease outbreak preparedness, 2018–2019. Global Health. 2020;16(1):24. Available from: https://globalizationandhealth.biomedcentral.com/articles/10.1186/s12992-020-00548-5. doi:10.1186/s12992-020-00548-5.
