Research | Open Access | Volume 8 (3): Article 60 | Published: 31 Jul 2025
Socio-economic burden of Rift Valley fever outbreak in a pastoralist community in Marsabit County, Kenya, 2018
Menu, Tables and Figures
Navigate this article
Tables
| Variables | Number | Percent | Wards | |
|---|---|---|---|---|
| Laisamis | Logologo | |||
| Age group in years | ||||
| 10–19 | 9 | 2 | 6 | 3 |
| 20–29 | 79 | 21 | 46 | 33 |
| 30–39 | 58 | 15 | 34 | 24 |
| 40–49 | 145 | 38 | 76 | 69 |
| 50–59 | 66 | 17 | 20 | 46 |
| >60 | 27 | 7 | 10 | 17 |
| Sex | ||||
| Male | 126 | 33 | 46 | 80 |
| Female | 258 | 67 | 146 | 112 |
| Marital status | ||||
| Married | 298 | 78 | 157 | 141 |
| Widow | 52 | 13 | 23 | 29 |
| Divorced | 12 | 3 | 10 | 2 |
| Single | 22 | 6 | 2 | 20 |
| Religion | ||||
| Christian | 347 | 90 | 174 | 173 |
| Muslim | 25 | 7 | 6 | 19 |
| Others | 12 | 3 | 12 | 0 |
| Level of education | ||||
| No education | 248 | 65 | 121 | 127 |
| Primary | 54 | 14 | 33 | 21 |
| Secondary | 38 | 10 | 20 | 18 |
| College | 35 | 9 | 18 | 17 |
| Adult education | 9 | 2 | 0 | 9 |
| Occupation | ||||
| Livestock keeper | 331 | 86 | 180 | 151 |
| Crop Farming | 9 | 2 | 8 | 1 |
| Self-employed Business | 26 | 7 | 3 | 23 |
| Student | 18 | 5 | 1 | 17 |
Table 1: Demographic characteristics of herd owners in Laisamis Sub-county during the RVF outbreak, N=384
| Species | Laisamis Ward n (%) | Logologo Ward n (%) | Overall Owned N (%) | Number of Deaths | Species-specific Mortality Rate |
|---|---|---|---|---|---|
| Goats | 5691 (35.5%) | 10,355 (64.5%) | 16,046 (45.6%) | 3,486 | 21.7% |
| Sheep | 5,721 (46.8%) | 6,491 (53.2%) | 12,212 (34.7%) | 2,735 | 22.4% |
| Cattle | 1,856 (43.5%) | 2,407 (56.5%) | 4,263 (12.1%) | 3,097 | 72.6% |
| Camel | 1,012 (38%) | 1,648 (62%) | 2,660 (7.6%) | 1,575 | 59.2% |
| Total | 14,280 (40.5%) | 20,901 (59.5%) | 35,181 (100%) | 10,893 | 31.0% |
Table 2: Proportions of livestock species owned by different herders in Laisamis Sub-county during RVF outbreak
| Species | Herd size prior to outbreak | Herd size after | Deaths | % (-ve) change |
|---|---|---|---|---|
| Camels | 4235 | 2735 | 1575 | 37.2 |
| Cattle | 7360 | 4263 | 3097 | 42.1 |
| Goats | 19532 | 16046 | 3486 | 17.8 |
| Sheep | 14737 | 12172 | 2735 | 18.5 |
Table 3: Livestock species and their respective herd reduction proportion after the outbreak
| Characteristic | Total N=384 | Laisamis n (%) | Logologo n (%) |
|---|---|---|---|
| Symptom | |||
| Stormy Abortions | 382 (99.5%) | 190 (49.4%) | 192 (50.3%) |
| Bleeding | 157 (40.9%) | 4 (2.5%) | 153 (97.5%) |
| Neurological signs | 111 (28.9%) | 5 (4.5%) | 106 (95.5%) |
| Reasons for keeping livestock | |||
| Sale | 368 (96%) | 180 (48.9%) | 188 (51.1%) |
| Slaughter | 375 (97.9%) | 190 (50.6%) | 185 (49.4%) |
| Milk production | 380 (98.9%) | 150 (39.7%) | 230 (60.3%) |
| Prestige | 0 | 0 | 0 |
| Not sure | 0 | 0 | 0 |
| Protection measures taken | |||
| Yes | 336 (87.5%) | 189 (56.2%) | 147 (43.8%) |
| Types of protective measures taken | |||
| Treat themselves | 380 (98.9%) | 191 (50.3%) | 189 (49.7%) |
| Veterinarian | 52 (13.5%) | 0 (0.0%) | 52 (100%) |
| Herbal treated | 34 (8.8%) | 2 (5.8%) | 32 (94.2%) |
| Vaccinated | 24 (6.2%) | 1 (4.1%) | 23 (95.9%) |
Table 4: Proportion of herd owners reporting herd syndromes, protection measures taken and reasons for keeping livestock in Laisamis Sub-County, 2018 (N=384)
| Species | Losses due to livestock mortality | Milk losses due to mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Category | Price (Ksh)/species | Total Direct Losses (Ksh) | Number of Lactating Dead Females | Price/liter (Ksh) | Liters/day | Duration (Days) | Total (Ksh) | |
| Camels | Adult Male | 60,000 | 20,100,000 | |||||
| Adult Female | 50,000 | 32,500,000 | 399 | 80 | 4 | 60 | 7,660,800 (59.6%) | |
| Young ones | 15,000 | 8,850,000 | ||||||
| Subtotal (1575) | 61,450,000 (38.9%) | |||||||
| Cattle | Adult Male | 50,000 | 10,700,000 | |||||
| Adult Female | 30,000 | 58,680,000 | 253 | 70 | 3 | 60 | 3,187,800 (24.8%) | |
| Young ones | 15,000 | 13,905,000 | ||||||
| Subtotal (3097) | 83,285,000 (52.8%) | |||||||
| Goats | Adult Male | 5,000 | 2,490,000 | |||||
| Adult Female | 3,500 | 3,447,500 | 671 | 100 | 0.5 | 60 | 2,013,000 (15.7%) | |
| Young ones | 500 | 1,001,500 | ||||||
| Subtotal (3486) | 6,939,000 (4.4%) | |||||||
| Sheep | Adult Male | 4,000 | 1,068,000 | |||||
| Adult Female | 3,500 | 4,438,000 | 1143 | N/A | N/A | N/A | N/A | |
| Young ones | 500 | 600,000 | ||||||
| Subtotal (2735) | 6,106,000 (3.9%) | |||||||
| Total (10,893) | 157,780,000 | 12,861,600 | ||||||
| Grand Total (Ksh) | 170,641,600 | |||||||
Ksh: Kenya Shillings
Table 5: Economic losses from livestock mortality and milk losses related to female livestock deaths in Laisamis Sub-county during the RVF outbreak in 2018
| Category | Amount (KSH) |
|---|---|
| Mortality losses | 157,780,000 (80%) |
| Milk losses due to mortality | 12,861,600 (6.6%) |
| Abortion losses – abortus/fetus | 10,966,500 (5.5%) |
| Subtotal (Direct loss) | 181,608,100 (92.1%) |
| Potential milk losses from aborting animals | 14,580,000 (7.4%) |
| Other losses/expenses* | 1,069,000 (0.5%) |
| Subtotal (Indirect loss) | 15,649,000 (7.9%) |
| Total | 197,257,100 |
*Other losses include: consultation costs, purchase of drugs, slaughtering or burying sick animal carcasses
Ksh: Kenya Shillings
Table 6: Summary of monetary losses incurred by herd owners in Laisamis Sub-county during the RVF outbreak, 2018
| Species | Losses due to abortions | Potential milk losses due to abortions | Total losses due to abortions and milk losses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Cost (Ksh)/abortion | Total loss (Ksh) | Number | Price/litre (Ksh) | Litre/day | Days of lactation | Total loss (Ksh) | ||
| Camel | 381 (11.7%) | 15,000 | 5,715,000 (52.1%) | 381 | 80 | 4 | 60 | 7,315,200 (50.1%) | 13,030,200 |
| Cattle | 263 (8.1%) | 15,000 | 3,945,000 (36.0%) | 263 | 70 | 3 | 60 | 3,313,800 (22.7%) | 7,258,800 |
| Goats | 1317 (40.4%) | 500 | 658,500 (6.0%) | 1317 | 100 | 0.5 | 60 | 3,951,000 (27.1%) | 4,609,500 |
| Sheep | 1296 (39.8%) | 500 | 648,000 (5.9%) | 1296 | 0 | 0.5 | 60 | 0 (0.0%) | 648,000 |
| Total | 3257 | 10,966,500 | 14,580,000 | 25,546,500 | |||||
Ksh: Kenya Shillings
Table 7: Losses due to abortions and potential milk losses from the aborting animals in Laisamis Sub-county during the RVF outbreak
| Variables | Categories | Economic loss n (%) | Odds Ratio | 95% Confidence Interval | P value |
|---|---|---|---|---|---|
| Age groups (years) | 10 – 19 | 7 (5.3) | 15.4 | 2.4-97.7 | (0.004) |
| 20 -29 | 28 (21.4) | 2.41 | 0.83-7.08 | (0.108) | |
| 30 -39 | 13 (9.9) | 1.27 | 0.40 – 4.02 | (0.683) | |
| 40 -49 | 63 (48.1) | 3.38 | 1.21-9.42 | (0.019) | |
| 50-59 | 15 (11.4) | 1.29 | 0.42-4.00 | 0.654 | |
| 60+ | 5 (3.8) | Reference | constant | ||
| Sex | Male | 60 (45.8) | 2.39 | 1.54-3.73 | 0.001 |
| Female | 71(54.2) | Reference | |||
| Marital status | Married | 107(81.7) | Reference | Constant | |
| Widow | 4 (3.0) | 0.15 | 0.05-0.42 | 0.0004 | |
| Divorced | 5 (3.8) | 1.28 | 0.39-4.11 | 0.684 | |
| Single | 15 (11.4) | 3.83 | 1.51-9.67 | 0.05 | |
| Religion | Christian | 122 (93.1) | Reference | Constant | |
| Muslim | 9 (6.9) | 1.18 | 0.51-2.74 | 0.707 | |
| Others | 0 | 0.08 | 0.005-1.421 | 0.086 | |
| Education level | No education | 82 (62.6) | Reference | Constant | |
| Primary education | 12 (9.2) | 0.58 | 0.29-1.16 | 0.12 | |
| Secondary | 16 (12.2) | 1.47 | 0.73-2.95 | 0.28 | |
| College | 13 (9.9) | 1.19 | 0.57-2.49 | 0.63 | |
| Adult education | 8 (6.1) | 16.19 | 1.99-131.68 | 0.009 | |
| Occupation | Livestock keeper | 106 (80.9) | Reference | Constant | |
| Crop Farming | 0 (0) | 0.13 | 0.007-2.189 | 0.155 | |
| Self-employed Business | 14 (10.7) | 2.81 | 1.26-6.27 | 0.014 | |
| Student | 11 (8.4) | 3.78 | 1.43-10.02 | 0.008 | |
Table 8: Relationship of Social demographic characteristics and economic losses of herd owners in Laisamis Sub-county during the RVF outbreak, 2018
Figures


Keywords
- Socioeconomic burden
- Rift Valley fever outbreak
- Direct and indirect monetary losses
- Herd owners
Mathew Munyamaara Mutiiria1,2,3, Peter Gatongi1, Elvis Oyugi4, Mathew Muturi3, Athman Mwatondo3, Bernard Chege5, Juster Mungiiria6
1Moi University School of Public Health, Eldoret, Kenya, 2Field Epidemiology and Laboratory Training Programme, MOH, Kenya, 3Zoonotic Diseases Unit (One Health office), Kenya, 4Division of National Malaria Programme (DNMP), Ministry of Health, Kenya, 5County Government of Marsabit, Kenya, 6Department of Biological Sciences, Chuka University, Chuka, Kenya
&Corresponding author: Mathew Munyamaara Mutiiria, Moi University School of Public Health, Eldoret, Kenya, Email: mathewmutiiria@gmail.com, ORCID: https://orcid.org/0000-0002-1888-4663
Received: 03 Dec 2024, Accepted: 31 Jul 2025, Published: 31 Jul 2025
Domain: Health Economics, One Health
Keywords: Socioeconomic burden, Rift Valley fever outbreak, Direct and indirect monetary losses, Herd owners
©Mathew Munyamaara Mutiiria et al. Journal of Interventional Epidemiology and Public Health (ISSN: 2664-2824). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Mathew Munyamaara Mutiiria et al., Socio-economic burden of Rift Valley fever outbreak in a pastoralist community in Marsabit County, Kenya, 2018. Journal of Interventional Epidemiology and Public Health. 2025;8(3):60. https://doi.org/10.37432/jieph-d-24-02002
Abstract
Introduction: Rift Valley Fever (RFV) is an acute vector-borne viral zoonotic notifiable disease primarily of domestic animals. It causes significant economic impacts among livestock producers and consumers. An outbreak of RVF occurred in May-June 2018; Marsabit reported positive cases in humans and animals. The study described herd owners’ social demographic characteristics, estimated livestock mortality and quantified the direct and indirect monetary losses in Laisamis Sub-County.
Methods: We conducted a cross-sectional study. Data was collected from 384 households. Direct and indirect costs were estimated using the economic model as described in Velthuis et al. (2008). Descriptive statistics were used to describe social-demographic characteristics. Economic burden incurred was the outcome variable.
Results: Majority of the respondents were females (67.2%). Total direct and indirect economic burden/losses were 1,970,000 USD, cattle recorded the highest financial loss at 42.3% (832,850 USD) through mortality. Stormy abortions were the most common syndrome reported at 382(99%).No routine livestock vaccination against RVF. Young herd owners were more likely to suffer economic losses compared to the aged (>60 years of age). Male herd owners and those with formal education were less likely to experience financial loss at (OR=0.42, p-value < 0.0001) than female herd owners.
Conclusion: A higher economic burden was associated with the RVF outbreak in Laisamis, particularly among female-headed households of younger age groups. Men had less odds of experiencing a financial burden compared to women. There is a need to vaccinate animals against RVF at the local level to reduce economic losses associated with an outbreak.
Introduction
Rift Valley Fever (RVF) is an acute, vector-borne, viral zoonotic notifiable disease affecting domestic animals and humans [1]. It is a re-emerging zoonotic disease whose distribution in sub-Saharan Africa and the Arabian Peninsula is widely reported [2]. RVF disease in animals usually starts with subclinical signs like fevers, cough, and runny nostrils. The disease can progress and form complications such as abortions, fetal malformation and subclinical to fatal febrile illness.
Globally, livestock production plays a vital role in the economic development of many pastoralist communities, particularly low-income families [3]. Apart from the effect on the economy, the disease also affects the health of people and animals. In human beings, RVF primarily causes a self-limiting febrile illness in the majority of the population. However, a small proportion of cases progress to develop hemorrhagic, neurologic and ocular complications [4]. The case fatality rate for severely complicated RVF is as high as 91% [5], while for moderate/mild cases, the fatality as low as 6% [6].
The disease is enzootic in sub-Saharan Africa, and specifically, RVF outbreaks have occurred across Eastern Africa from 1912 to 2010, approximately every 4 –15 years [7]. For example, in Tanzania, the disease has been causing outbreaks since 1931 up to 2007, when the last major outbreak took place [8]. It was associated with an increased vector population because of flooding following heavy rainfalls in many flood-prone habitations, creating a conducive habitat for breeding. Aedes spp, Culex spp and other mosquito species are important epidemic vectors for this disease. Nomadic pastoralists and agro-pastoralist communities are at high risk of contracting the disease during epidemics. This is due to poor living conditions coupled with a lack of knowledge on the epidemiology of RVF, therefore increasing the chances of exposure to the disease [9].
In Kenya, RVF outbreaks have previously been reported in several counties such as Tana River, Garissa, Isiolo and Lamu. Ecological factors have been identified to be a risk factor for the occurrence of some outbreaks [10]. Additionally, outbreaks in Kenya are episodic and are related to climate variability, especially rainfall and flooding [11].
In domestic animals, the transmission of RVF is either through bites from different species of infected mosquitoes, mainly the Aedes and Culex genera, or direct contact with infected animal tissues, body fluids, and fomites causes transmission in humans [12]. The Aedes mosquitoes are the primary vectors of RVF in animals. These mosquitoes maintain the virus transovarially; the virus is spread from the parent to offspring via infection of the developing egg, which subsequently results in infectious adult mosquitoes. Also, during drought, infected mosquitoes lay eggs resistant to harsh weather that survive in dry soils on low-lying depressions until the rains return [13]. On the other hand, transmission to humans is via direct contact. This is where consumption of infected animal products, such as milk or meat, causes a transmission of the disease; contact with aborted fetuses and from bites of infected mosquitoes, most commonly the Aedes species [4].
RVF disease outbreaks cause great socioeconomic losses in livestock sectors [14]. The economic impacts of a disease can be defined as the consequence of a disease occurrence and its management, control, prevention and surveillance costs[15]. The economic impact of RVF can be classified into direct or indirect costs [16]. Some direct costs incurred during such outbreaks include: the cost of managing the disease, buying larvicides, the cost of disposal in the event of a death and, cost of surveillance. On the other hand, indirect losses include human morbidity, livestock mortality, loss of milk production, low weight gains and abortions; a significant economic disruption mainly due to livestock losses and trade restrictions[13].
Livestock keeping is the basis of human subsistence and prosperity for pastoralist communities, as well as a cultural life and social organization [17]. It represents an important livelihood function by providing valuable goods and services such as milk, meat, blood, manure, transport and financial services.
The impact of livestock diseases on agriculture is usually assessed in quantitative terms: lost revenues, cost of eradication, decontamination, vaccination and restocking, and the numbers of affected farms, animals and humans [18]. In the event of an RVF outbreak, producers and livestock industries are usually affected. Moreover, public and animal health workers, food security and the livelihood of the pastoralist communities are significantly affected[19].
Zoonotic disease outbreaks constitute a significant threat to livestock farming-based economies. In 2016, animal diseases such as the highly pathogenic avian influenza (HPAI) H5N1 affected the world’s economy with estimated losses in billions of US dollars worldwide and economic impact equivalent to 2% of the East Asian gross domestic product (GDP)[20]. Consequently, HPAI has become a major focus point worldwide due to its zoonotic potential and economic effects resulting from trade restrictions and high mortality rates in poultry [20].
Equally, the RVF outbreak causes a major threat to the economies of many countries and regions as it affects food security. The agricultural sector suffers the most negative impacts of a RVF outbreak.
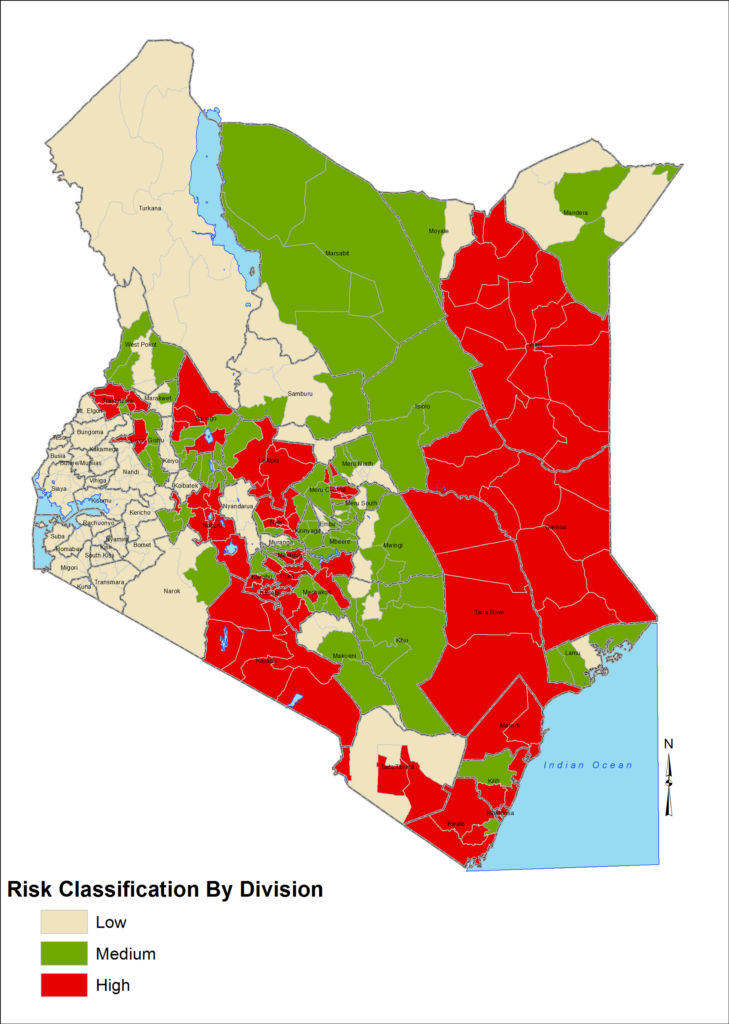
An outbreak of RVF was confirmed in Kenya in June 2018 in some of the high-risk counties (Figure 1). This outbreak was picked through the enhanced syndromic surveillance (ESS) system that had begun in May 2018 from the Zoonotic Disease Unit (ZDU). This surveillance system was activated due to the alerts from the meteorological department, which were earlier issued in April 2018. About ten [10] counties reported confirmed cases of animal and human RVF outbreaks. Marsabit County was one of the counties that experienced the outbreak among other counties like Wajir, Tana River, and Mandera under surveillance. The disease posed a great threat not only to the livestock keepers but also to the Government due to its social and economic implications. Some costs were incurred on measures taken at different levels in order to prevent or control the disease outbreaks in Marsabit County. The RVF outbreak affected people both directly and indirectly. Directly, livestock keepers lost their animals through deaths and massive abortions. Approximately over thirty thousand livestock deaths were reported in Marsabit County. Indirect losses included the lack of meat for people to eat during the ban on livestock movement and slaughter and the loss of a livelihood for those who depended on livestock. In addition, herders incurred costs such as vaccination and consultation fees when their animals fell sick.
While livestock production plays an increasingly important role in the economies of many pastoral communities, it remains vulnerable to many diseases[21]. Losses of animals were widespread during the RVF outbreak in Marsabit County. Considering the current zoonotic threat of RVF and the increasing risk of spread to some disease-free countries, there is a need for better understanding of the socio-economic impact of RVF.
Despite the knowledge that the previous RVF outbreaks affected farmers negatively, no sufficient quantitative assessment has been conducted to establish the extent of this impact on farmers’ herds. This study sought to quantify the economic burden of RVF following the outbreak in the Laisamis in Marsabit County, Kenya. This information will be useful to both farmers and the county government of Marsabit by providing data which could be useful for planning purposes. This will result in evidence-based recommendations on control options for RVF from the societal perspective and possibly improve response capacity to future RVF outbreaks in the county as well as at the national level. The objectives of this study were: to describe the social demographic characteristics of herd owners affected by the 2018 RVF outbreak, estimate livestock mortality, quantify the monetary losses attributable to the RVF outbreak and describe factors associated with the economic burden of the outbreak in Laisamis Sub-County.
Methods
Study site
The study was conducted in the Laisamis Sub-County, Marsabit County. The County is located in Northern Kenya, borders Turkana County to the west, Samburu County to the south, and Wajir and Isiolo counties to the east. It’s divided into four sub-counties: Saku, Laisamis, North Horr, and Moyale. This is one of the counties that experienced an outbreak of RVF in 2018, and the study was conducted in October 2021. Marsabit County had an approximate population of 291,166 people, with a ratio of 52% for males and 48% for females.. The most common religions are Islam (40%) and Christianity (32%), and 28% have other religious affiliations[22].
As is the case with the larger part of Marsabit County, the main economic activity is livestock production practised mainly extensively under a nomadic pastoral production system[22]. There exist two main active livestock markets, i.e., Merille and Loyangalani, that are most accessible for livestock producers and traders. Trading centres like Merille, located along the Isiolo-Marsabit highway, have relatively vibrant economic activity for the area and are increasingly well-connected to growing mobile phone service coverage.
Study design
A cross-sectional study design was used to elicit information on the economic loss incurred by the pastoralists in Laisamis Sub-County. Data for this study were collected in October 2021. The cost survey analysis approach was applied to estimate actual costs/losses from individual herd owners. This approach captures the variability of the expenses/losses among herd owners. To collect data on financial losses/costs, a modified deterministic economic model by Velthuis et al. (2008) was used. The model proposes the following formula:
$$L = \sum_i \sum_j P_{i.j} + T_{i.J} + D_i + M_{i.J}$$
Where:
L represents the total economic loss in the livestock production due to the outbreak,
P I J is the monetary losses by type of farming and type of Species – J,
T is the corresponding treatment cost per animal type and farming type
D is the diagnostic cost per farming type and species
M is the cost of control measures per farming type and species
Producer’s (herder) node losses: We only considered the livestock value chain at the producer’s node. Thus, i.e., Mortality (MT), abortions (AB), and milk production (MP)
Where
MT= ad (sv)
Where ad –number of animals dead multiplied by the sv-slaughter value for each of the species,
For abortion losses, the formula was as follows: –
AB = ab (bv) abortion
Where ab is the number of abortions multiplied by “bv” is the cost of the animal at birth.
To estimate the milk production losses, the following formula was used:
$$MP_j = mbr_j \cdot rp(ar_j)(amp_j)(rmp_j)^{0.5}(dd_j)(VM_j) \div 100$$
Where:
“mbr” is the morbidity rate per 100 animal months,
“rp” is the period at risk per species,
“ar” is the total number of animals per species,
“amp. j” is the average dairy production per species,
“rm. pj” is the relative reduction in milk production per species,
0.5 it is the assumption that milk production is reduced during the first half of this period
“dd” the duration (in days) that the animal is down with the disease;
“vm’ is the value of milk produced per species
From the key informant’s interview data information, the researchers were able to calculate other costs/losses that livestock farmers incurred during the RVF outbreak in the Laisamis sub-county
Inclusion and Exclusion criteria
The inclusion criteria were all households that own livestock of any type, regardless of whether they experienced any form of loss or not were eligible. Under exclusion criteria, any herd owner who did not provide consent or all household owners who were not residents of Laisamis Sub-County before 2018 were excluded. This is because data collection was done two years after the outbreak occurred and the study was meant to estimate the economic burden experienced by the people (respondents) who lived in that area during the outbreak of RVF .
Dependent variable
This was Economic losses- YES/NO. The binary outcomes were determined by calculating the mean economic loss from all the losses among the herd owners. Herd owners whose economic losses were below the mean were considered to have incurred minimal economic losses and were assessed and denoted by “No.” Those whose economic losses were above the mean were considered to have incurred significant monetary losses and were designated “Yes.”
Sample size determination:
The sample size was determined using the Cochran formula (1977) for quantitative data. This is where we assumed that at least 50% of the population came down with the disease and that the remaining 50% did not have an outbreak of RVF.
sample size
$$n = \frac{z^2 \cdot p \cdot q}{e^2}$$
Where n is the expected minimum sample
z score = 1.96
p = 0.5% (estimated RVF prevalence)
q is the proportion without RVF outbreak =0.5 (1-0.5)
e2 = the standard error =0.05
Therefore, our sample size was: –
n =1.962(0.5)(1-0.5)÷0.052
n = 3.8416*0.25÷0.0025
=0.9604÷0.0025=384.16
n = 384
Sampling procedure:
Sub-County Veterinary officers and animal health assistants helped in mapping herd owners from Laisamis and Logologo wards. Twenty geocodes were randomly generated from the two wards. Upon generating twenty central geocodes, we located each central geocode and systematically picked every second household up to ten households in one direction, then back to the main point and moved to the opposite direction until we obtained ten households. This process was repeated in the other 19 geocodes.
The nth household was determined from the estimated population of the households in the two wards divided by the calculated sample size. Further, the sample size was divided by the twenty days allocated for data collection. Each central geocode was to be covered per day. This was repeated in other central geocodes until three hundred eight four (384) households were obtained. In a household where the herd owner was absent, the team moved to the next household or came back the following day, especially if information was available that the herd owners could be available the following day.
Data collection
The household survey was collected by conducting face-to-face interviews using structured electronic questionnaires in Epi Info. The questionnaire was administered to herd owners. Data on demographic information, farming practices, prevention of RVF, animal mortality, and abortions were collected, among other variables. The questionnaire included open-ended questions to collect qualitative data as well as close-ended questions to collect quantitative data.
Data on the monetary values of the livestock products was obtained from key informant’s interviews. They included Sub-County livestock production officers (SLPO), animal health assistants, and area chiefs. For related market variables in the County, for example, researchers obtained average product prices, input prices, and RVF diagnostics performed from key informants’ interviews.
Data management and statistical analysis
The data was collected using an electronic questionnaire loaded into mobile phones and tablets. The collected data was downloaded as a Microsoft Excel database and then exported to SPSS version 25 for analysis. Descriptive statistics on the sample characteristics and questionnaire items were computed, including frequency distributions. We utilized the cost-of-survey approach to get actual costs/losses from individual herd owners. The cost of survey approach can capture the variability of the expenses/losses among herd owners. A chi-square test was conducted to determine the association between demographic variables and economic burden. Inferential statistics was calculated at a 95% confidence interval with a precision level of p< 0.05. Additionally, multivariable logistic regression model was conducted to determine the relationship between the outcome variable (economic loss) and independent variables.
Ethical considerations
Ethical clearance was sought from Moi University Institutional Review and Ethics Committee (IREC) reference number IREC2019/207. Permission to carry out the research was sought from Marsabit County government department of Veterinary Services Ministry of Agriculture, Livestock and Fisheries. Participant consent was obtained from the eligible herders who were participating in the study. An explanation as to why the study was being carried out was provided to the participants. Respondents were allowed the freedom to opt out of the interview without being subjected to any consequence of withdrawing from the study.
Results
Socio-demographic characteristics of herd owners: A total of 384 household heads were interviewed. The median age of the household head was 40 years, with an interquartile range (IQR:17 – 84 years). Of the respondents interviewed, 77.6% (298/384) were married, and 67.2 % (258/384) were female. On the other hand, the majority, 248 (64.7%) of the household heads, had no formal education, while only 73 (19%) of the respondents had secondary education or above. (Table 1) The primary occupation of the respondents was livestock keeping, 331 (86.2%), while less than 10% practiced mixed types of farming. Livestock keepers in this study were drawn from five locations in the two wards, i.e., Logologo and Laisamis. These were picked in equal proportions – each 192, since the number of herd owners in these two wards was almost similar, as per the ward veterinary officer data.
Livestock species kept and herd size reduction: The most commonly kept species were goats, at 45.6% (16046/35,181), followed by sheep at 34.7% (12,212/35,181). Logologo ward had the highest number of goats at 64.5% (10,355/16,046) and sheep at 53.2% (6,491/12,212). (Table 2) On the other hand, the commonest species in Laisamis ward was sheep at 40.1% (5,721/14,280). In our study, herd size reduction was an involuntary reduction of the livestock due to the RVF outbreak. The overall herd size reduction due to the RVF outbreak was 23.6%(10893/46074) The highest reduction was in cattle at 42.2% (3097/7360), followed by camels at 37.2% (1575/4235), while goats and sheep had the lowest herd size reduction at 17.8% (3486/19532) and 18.6% (2735/14737), respectively (Table 3)
Clinical syndromes reported and interventions taken by herd owners: We sought to understand the common symptoms that were reported during the outbreak of RVF in Laisamis Sub-county (Table 4). Most of the herd owners, 382 (99.5%), reported stormy abortion among their herds, while neurological symptoms were the least reported at 111 (28.9%).
On whether herd owners intervened and the type of interventions undertaken, 87.5% (336/384) intervened, with 52 (13.5%) of the respondents hiring the services of a veterinarian, while almost an equal proportion, 34 (8.8%), used herbal intervention during the outbreak. Those who sought or received the services of a veterinary officer were all from the Logologo ward. The majority of those who used herbal medication to treat their livestock during the outbreak resided in Logologo. (Table 4)
Economic losses from livestock mortality: The key informant interviewees provided the estimated values for different livestock species and livestock products in Kenyan shillings. These values were used to calculate the various types of losses in the study.
The economic losses incurred by herders during the outbreak were classified into direct and indirect losses. Direct economic losses calculated in this study were losses from the different livestock species associated with animal mortality, losses due to abortions and loss of milk production from the dead adult female animals that were lactating for two months after the outbreak. Total direct losses during the study period were Kenya shillings 181,608,100. Losses due to livestock mortality and milk losses associated with dead adult female animals amounted to Kenya shillings 170, 641,600 with livestock mortality accounting for over 92% (157,780,000/170,641,600) (Table 5). Sheep were not kept for milk production; therefore, no prices were indicated for milk from sheep since this was not being sold in the community. Camels contributed 60% (7,660,800/12,861,600) of the milk losses. Total livestock mortality contributed over 86.9% (157,780,000/181608100) of the direct economic losses that herd owners incurred during this outbreak.
The total indirect losses from all the livestock species in this study comprised of the potential milk losses associated with the abortions plus additional costs incurred to avoid or reduce disease spread, such as purchasing drugs, and slaughtering or burying sick animal carcasses. This was computed to be Ksh 15,649,000 and accounted for 7.9% of all economic losses incurred (Table 6). Notably, the total losses due to abortions and potential milk losses associated with abortions amounted to Ksh 25,546,500. However, the economic losses related to abortions were slightly lower (43%, 10,966,500/25,546,500) than the losses related to potential milk losses (Table 7). The most affected species in terms of abortions was the goat at 1317 (40.4%), while the cattle were the least affected 263 (8.1%). However, looking at the economic losses related to foetal wastage alone, camels had the highest loss of Ksh 5,715,000 (52.1%), while sheep had the least Ksh 648,000 (5.9%). The camel species had the highest potential milk loss at KSh 7,315,200 (50.1%), Table 6.
Summary of losses
Almost eighty per cent (80%) of the losses were mainly from livestock mortality (Table 6), whereas other types of losses had minimal economic loss. The total economic loss from livestock mortality, milk loss due to livestock mortality, abortions, milk losses due to abortions, and other expenses was Ksh 197,257,100 which is equivalent to approximately 1,970,000 US dollars. The assumed exchange rate was KSh 100/US. However, in terms of the proportion of the amount of money lost due to mortality, cattle contributed the highest loss at 52.8%, followed by camels at 38.9%. The economic losses in the small ruminants were 4.4% in goats, while sheep were 3.9% of the total deaths (Table 5).
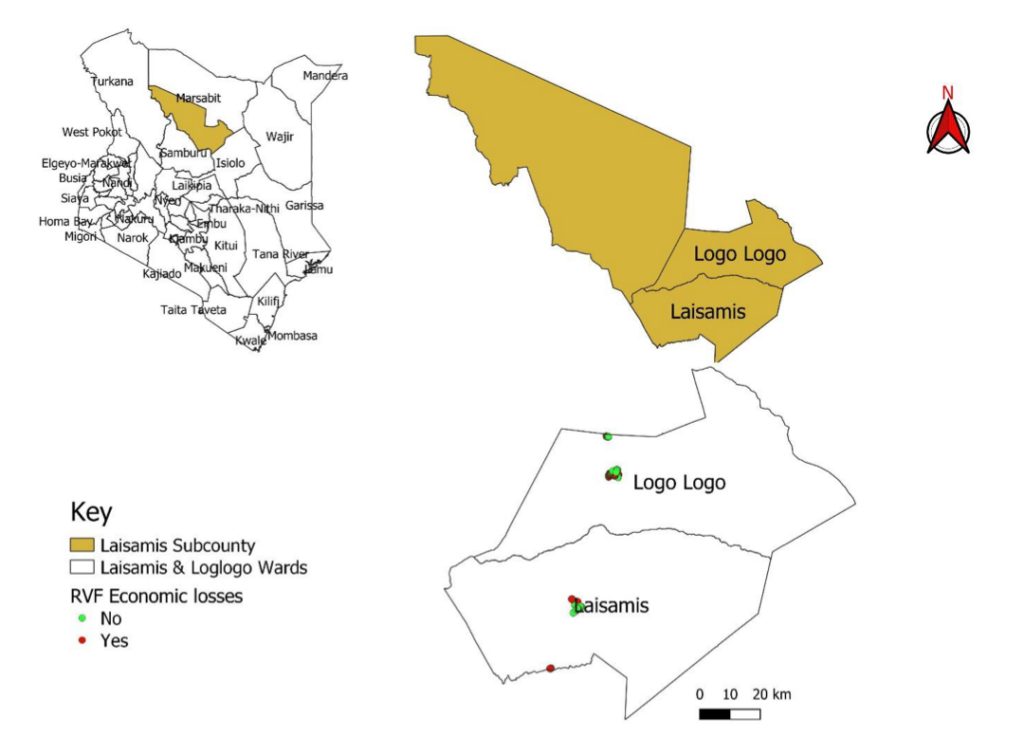
Economic losses among herd owners in Laisamis Sub-County
After calculating the economic losses, we plotted the households that incurred economic losses on the map of the Laisamis Sub-County. Figure 2. Most of the losses were found in the Logologo ward.
Factors associated with economic losses in Laisamis Sub-County
An estimated 34.1% (131/384) of the herd owners incurred significant monetary loss. Age, sex, marital status, education level and occupation were statistically significantly associated with economic losses (Table 8). The odds of incurring economic losses among herd owners in the lowest age group, 10-19 years, were 15 times higher than among the 60+ age group (OR=15.4, 95%CI= 2.4 – 97.7, p-value of 0.004). There were also significantly higher odds of economic losses among the 40 -49 years age group (OR=3.38, 95%CI= 1.21-9.42) compared with the 60+ years. The odds of incurring an economic loss were significantly higher among males (OR=2.39 odds, 95%CI=1.54-3.73) than females and among those who had attained adult education (OR=16.19, 95%CI=1.99-131.68) compared to those who had no education. Similarly, herd owners who were students (OR= 3.78, 95% CI=1.43-10.02) and self-employed (OR=2.81, 95% CI= 1.26-6.27) had higher odds of incurring economic losses compared to pry livestock keepers. Marital status was significantly associated with economic losses; compared with the married herd owners, those who were single had higher odds of incurring losses (OR=3.83, 95% CI=1.51-9.67) while the widowed had lower the odds (OR=0.15, 95%CI=0.05-0.42).
Discussion
This study sought to describe the social demographic characteristics of herd owners affected by the 2018 RVF outbreak, estimate the economic burden of this RVF outbreak and associated factors in Laisamis Sub-county. We found out that most of the herd owners were females, middle-aged (with a median age of 40 years, IQR:17-84 ), married, Christians, had no education and were students and livestock keepers as a primary occupation.
The predominance of females could probably be due to the active engagement of women in livestock activities among the pastoralist communities, especially in handling small livestock. These findings concur with a study by Muga et al [16] who found that women in sub-Saharan Africa frequently spent more time looking after the herds than their husbands, hence the proportion of women handling livestock was higher for women than men.
Livestock species kept and herd size reduction, Clinical syndromes reported & how herd owners intervened,
Stormy abortions are usually associated with RVF outbreak. In our study, almost all the respondents reported stormy abortions among their livestock herds. This observation was confirmed by the positive polymerase chain reaction (PCR) test results that were positive. These tests were used as the basis for confirmation of the RVF outbreak in the county. However, a study by Oyas and others established that only 36% of the respondents reported stormy abortion [23]. The difference in the proportions of stormy abortions in these two studies may be because the study by Oyas and others aimed to assess common RVF syndromes during the outbreak. Our study sought to explore the economic burden of outbreaks among livestock keepers, and therefore, we were more likely to pick up animal abortions because of their direct effect on the monetary value of the livestock in question.
Neurological signs were least reported by the respondents at 28.9%(111/394). These findings are unique in that A quantitative observational study conducted in the western region of Uganda involving blood samples and abortion events from 1000 livestock (goats, sheep and cattle) reported prevalence of abortion cases, but no neurological symptoms were reported or studied among animals that had RVF [24]. This same study also did not report on other syndromes reportable in the RVF outbreak. We therefore recommend conducting more studies on the major syndromes in an outbreak of RVF.
Protection measures taken Whereas the majority of the herd owners mentioned that they took some protective measures during the outbreak, less than a quarter of the livestock keepers utilised the services of a veterinary officer. Consequently, the majority of the respondents treated their animals themselves. This observation was also reported in a study by Page and Gautier (2012). In this study, the explanation for livestock keepers self-treating their animals was that they were afraid of losing their animals due to the disease by the time a para-veterinary officer comes to treat their animals. In so doing, it was observed that the economic losses were minimal [25].
Regarding the prevention of RVF outbreaks, in our study, most respondents felt that livestock vaccination was an important practice that needed to be adopted by many of the livestock keepers. According to a study by Gachohi et al (2016), the implementation of periodic biannual or annual vaccination of livestock has been shown to greatly reduce the risk of an outbreak [26]. Vaccination of livestock against RVF by qualified personnel has been cited as the most effective way to control RVF[27]. Since the disease causes huge economic losses and many negative public health impacts in endemic countries in Africa and the Arabian Peninsula, a proper vaccination strategy is an important approach for preventing or minimising outbreaks [28].
Herd size reduction
Due to the outbreak of RVF in the study sub-county, all the livestock species had a herd size reduction of about 23,6% It is important to note here that the herd size reduction was not due to herd dispersion but was due to death of the livestock, though in different proportions. Herd dispersion is a coping mechanism which pastoralist communities use to ensure that, once a disease strikes in a given area, all their livestock does not suffer the same effect of the disease and therefore end up saving some of them [16]. A study by El Mamy (2011) found that there was a herd size reduction following the unprecedented outbreak of RVF [29] in Northern Mauritania. However, that study did not indicate the exact figures in terms of herd size reduction. Perhaps this is because the study was not meant to establish the economic burden of the disease, but rather the change of herd size in a non-expected outbreak. Similarly, an economic assessment of zoonotic disease in the United States by Pandell et al (2016) only stated that there was an increase in mortality of livestock, but the number of livestock deaths was not stated, and hence the level of herd size reduction was not indicated [30]. A study conducted in Kenya showed that RVF-induced abortions in sheep resulted in a 22% reduction in potential flock size [31]
Economic losses incurred during RVF outbreak
RVF outbreak causes an important threat to a country/community[32]. The occurrence of an RVF outbreak means dramatic economic consequences for national and local economies, as well as the risk brought about by the disease spreading to other disease-free countries.
Our study determined that herd owners in Laisamis subcounty incurred an overall economic loss of Ksh 197 million (approximately USD 2 million), with about 92% attributed to direct losses ( Ksh 181 million ).
Direct and indirect losses
Mortality
The highest economic loss in this study was related to livestock mortality at 80%. Among the four major livestock species kept, cattle had the highest economic loss among the species considered. These findings resonate with a study in Tanzania on the epidemiology and socioeconomic impact of the RVF outbreak[33]. The study by Sindato et al (2012) estimated cattle mortality loss at 4,243,250 USD compared to our finding of about KSh. 83,285,000 (832,850 USD). The former was a country-wide economic assessment, whereas our study was conducted in one Sub-county. This could perhaps be the reason for the disparity in the findings.
Our findings are also inconsistent with those of a study by Rich &Wanyoike (2007), who reported a 32-million US dollar economic impact of RVF along the different livestock value chain actors and over USD9.3 million (Ksh 610 million) in total economic losses from livestock mortality in these two regions [31]. However, they pointed out that there was a negative impact on the producers in terms of food insecurity as well as reduced income. This observation compares to our study, which found an average loss of about Ksh 511,000 (5000USD) (USD1=100) per household from all the direct and indirect losses.
Contrary to our findings, a study by El Mamy (2011) indicated massive deaths of camels and a subsequent reduction in market prices by 40% [29], but the researcher did not quantify the amount lost in monetary terms.
Milk losses
In pastoral communities, such as Marsabit County, livestock keeping is the main source of livelihood. Milk provides benefits such as food and thus has high economic value to the residents [34]. In this study, by Ng’ang’a economic value of milk was not quantified. In our research, among other losses incurred by the livestock keepers in the Laisamis Sub-County were potential milk losses from the animals that had died and the potential milk losses from abortions, totalling Ksh 27,441,600 (equivalent to 274,416 US$) and accounting for 13.9% of the total economic losses. In another study by Peyre et al 2015, out of the many socioeconomic studies considered in the systematic review, no milk-specific economic loss was quantified [31]. According to a study done in Tanzania on the socioeconomic impact of RVF on the pastoralists, they cited average milk production sales per household to be 1-15 US dollars per day per household [12]. In our study in Marsabit County, the average economic loss due to milk lost per household in the two months was Ksh 33,500 (335 USD) in all the species, approximately 5 US dollars per day. The difference in this observation could have been due to the approaches used, whereby a socioeconomic impact of RVF was assessed, while in our case, we quantified the economic burden following the outbreak of RVF amongst the herd owners. The method that we used in quantification of the milk losses is likely to be different from what the researchers in Tanzania used; however, the difference in results is minimal, in that 5 USD is within the 1 to 15 USD range in Tanzania
In a study by Jemberu et al. (2014) in Ethiopia on the social, economic impact of foot and mouth disease amongst small-scale farmers, the study found that milk losses contributed the highest amount of loss among the small stock keepers. This appears different scenario in our study. Whereby mortality from livestock had the highest economic impact compared to other losses. These differences may be due to the type of the disease being evaluated. In addition to the above issue, mortality from big stocks such as cattle and camel in our case was high. However, the two diseases are trans boundary animal diseases (TAD). This Ethiopian study found that the economic loss per herd was KSh 7600 (76 US dollars), which is far different from our finding of Ksh 33,500 (335US dollars) per household in which, when translated to herds, would represent all the species under study.
Abortion losses during the outbreak
Our study has shown that losses related to abortion in livestock were 5.5% (KSh 10,966,500) of the total economic burden calculated. According to a study by Pendell et al (2016) on socioeconomic assessment of potential RVF outbreak in the United States, there was a significant negative impact among livestock keepers [30]. However, this study did not estimate the specific losses due to aborting animals. However, a study by Rich & Wanyoike [35] on the regional and national socio-economic impacts of the 2007 RVF outbreak in Kenya specifically cited milk losses from aborting camels as Ksh 758,800 in a period of four months. This significantly differs from our results of Ksh 7315200 (73152 USD) potential losses from aborting camels in Laisamis. It should be noted that their values were calculated for a period of about 4 months whereas in our case we considered the losses for only two months in which the outbreak lasted, We noted a herd size reduction of 23.6% Another study by Sindato et al (2012) stated that there were direct and indirect losses to farmers from the RVF outbreak but the study did not state or estimate the specific losses from abortions[31]. The study only showed results from mortality losses from cattle, sheep and goats. On the other hand, in a study in Tanzania on “socio-economic impact of RVF outbreak to pastoralists and agro-pastoralists”, the researcher did not state the losses related to abortion during the outbreak; rather, he only reported stormy abortions and 100% mortality [12]. This study reported economic activities without indicating the monetary values thereof.
Factors associated with economic loss.
In this study, livestock keepers experienced either minimal or high economic loss as these were the outcome variables. The total economic burden of the Rift Valley outbreak at the livestock value chain -producer node was approximately Kenya shillings 197,600,000 (1,976,000 USD). The results in this study have shown that gender played a significant role in determining the an economic loss or no economic loss. In our study, male herd owners had higher chances of economic losses compared to females. A study conducted in Sudan regarding social demographic determinants of RVF outbreak also found that the disease burden increased if the respondent was a male [36]. However, an in-depth understanding of the disease outbreak was lacking, just like in Laisamis, where the impact of the disease was not available until this study was conducted. On the other hand, a study by Muga et al [16] found that people’s behaviour is at times influenced by the economic incentives thereof, which is then linked to the gender roles of the community members. That study showed that African women are subjected to inferior economic status, consequently taking roles such as milking, unlike men who take the role of selling the livestock, thereby making them more economically empowered than their women counterparts [16].
In a study conducted in United States of America on assessment of economic impact of zoonotic diseases, the researchers did not consider economic losses per gender but instead did the assessment in economic impacts on agriculture-livestock producers cost of response to the outbreak by the government, cost of disruption by the outbreak and further considered human health costs such morbidity and mortality [30].
On the other hand this study has found out that the level of education was significantly associated with an economic loss. Herders who had attained adult education were 16 times more likely to have incurred economic losses compared to those who had no education. These findings are a special observation in that, according to a scoping study by Peyre, 17 studies identified for quantitative analysis relied only on partial cost analysis, with limited reference to mid- and long-term impact, public health or risk mitigation measures. However, the estimated impacts were high (ranging from USD 5 to USD 470 million losses) [31]. These studies did not analyse data on economic losses compared to the level of education, occupation of the herd owners ie either self- employed or being student. Therefore there is need to conduct more studies on the economic burden of RVF in relation to age, level of education and occupation so as to bring a comparison to our study findings Those herd owners in the lower age group 10-19, and middle aged group 40-49 were more likely to experience an economic loss than those in the 60+ age group. This observation is different from the study results conducted in Ethiopia on economic losses due to foot and mouth disease. The study only considered economic losses in age – sex strata, and they did not find a significant association between the economic losses to age groups [37]. The difference in these findings would be due to the different approaches used by the researchers. Consequently, we recommend future studies to cross-check the association of economic losses with other factors, like occupation subjected to a logistic regression model.
Study Limitations and Mitigation Measures
During the outbreak of RVF in Kenya in 2018, the exact number of livestock lost and monetary loss incurred by herd owners was not quantified by the county government or by any organization at that time. This study, therefore, relied on recall by herd owners and key informants to provide these details.
Some herd owners found it challenging to remember incidents surrounding the outbreak (recall bias). This is because the outbreak took place the previous two years. To mitigate this, we developed a standard local case definition of RVF so that the herd owners could easily understand the condition and describe the season or events surrounding the outbreak.
The other study limitation was the nomadic pastoral production system practised in the area. This led to herd owners becoming unavailable at home during data collection. The outcome of such scenarios was either underestimation or exaggerated results of losses. To manage this, for any household owner absent during the visit, we booked a repeat visit to ensure that we found them to increase their chances of participating in the study. Additionally, we worked with the local leaders/community members to help us identify the actual residents during the outbreak.
Conclusion
Most Livestock keepers in the Laisamis Ssub-Ccounty incurred a high economic burden during the RVF outbreak. The primary cause of the economic losses/burden was livestock mortality from the four major species. As a result of the livestock mortality, herd owners incurred an over all 23.6% herd size reduction per species. The most affected species was cattle, which had the highest economic burden.
The younger and middle aged herd owners experienced the majority of the losses, and male herd owners experienced more losses than female herd owners. The losses were also associated with adult education level, , and inadequate veterinary personnel, leading livestock keepers to use traditional means of managing the disease during the outbreak instead of seeking veterinary services. These study results can be applied by researchers to other settings with similar background characteristics to inform decision-makers of the need to invest more in disease prevention than control.
What is already known about the topic
- There is Minimal knowledge on the economic burden of the disease in the county
- RVF outbreak is usually preceded by flooding and high rainfall patterns
What this study adds
- Data is now available on the socioeconomic burden of RVF outbreak in one county in Kenya
- Information on herd size reduction has been provided, which other researchers can refer to
- Economic losses are associated with gender as well as age factors
Acknowledgements
I express deep appreciation to the Kenya Field Epidemiology and Laboratory Training Programme (K-FELTP) and the Zoonotic Diseases Unit (ZDU) in the Ministry of Health for granting me the opportunity to conduct my research together with my coauthors, additionally, they provided invaluable resources and unwavering support throughout the study period. Special thanks to Moi University School of Public Health, the County government of Marsabit for their moral support and encouragement. Lastly, profound gratitude to all the participants in my study whose time and willingness to share their experiences were indispensable. This work owes its completion to their invaluable contributions.
Authors´ contributions
The authors confirm their contribution to the paper as follows: study conception and design: PG, E.O; Data collection: – B.C; analysis and interpretation of results: – PG, B.C, J.M, E.O; draft manuscript preparation: – M.M, A.M, B.C. All authors reviewed the results and approved the responses to the reviewer’s comments on the manuscript.
| Variables | Number | Percent | Wards | |
|---|---|---|---|---|
| Laisamis | Logologo | |||
| Age group in years | ||||
| 10–19 | 9 | 2 | 6 | 3 |
| 20–29 | 79 | 21 | 46 | 33 |
| 30–39 | 58 | 15 | 34 | 24 |
| 40–49 | 145 | 38 | 76 | 69 |
| 50–59 | 66 | 17 | 20 | 46 |
| >60 | 27 | 7 | 10 | 17 |
| Sex | ||||
| Male | 126 | 33 | 46 | 80 |
| Female | 258 | 67 | 146 | 112 |
| Marital status | ||||
| Married | 298 | 78 | 157 | 141 |
| Widow | 52 | 13 | 23 | 29 |
| Divorced | 12 | 3 | 10 | 2 |
| Single | 22 | 6 | 2 | 20 |
| Religion | ||||
| Christian | 347 | 90 | 174 | 173 |
| Muslim | 25 | 7 | 6 | 19 |
| Others | 12 | 3 | 12 | 0 |
| Level of education | ||||
| No education | 248 | 65 | 121 | 127 |
| Primary | 54 | 14 | 33 | 21 |
| Secondary | 38 | 10 | 20 | 18 |
| College | 35 | 9 | 18 | 17 |
| Adult education | 9 | 2 | 0 | 9 |
| Occupation | ||||
| Livestock keeper | 331 | 86 | 180 | 151 |
| Crop Farming | 9 | 2 | 8 | 1 |
| Self-employed Business | 26 | 7 | 3 | 23 |
| Student | 18 | 5 | 1 | 17 |
| Species | Laisamis Ward n (%) | Logologo Ward n (%) | Overall Owned N (%) | Number of Deaths | Species-specific Mortality Rate |
|---|---|---|---|---|---|
| Goats | 5691 (35.5%) | 10,355 (64.5%) | 16,046 (45.6%) | 3,486 | 21.7% |
| Sheep | 5,721 (46.8%) | 6,491 (53.2%) | 12,212 (34.7%) | 2,735 | 22.4% |
| Cattle | 1,856 (43.5%) | 2,407 (56.5%) | 4,263 (12.1%) | 3,097 | 72.6% |
| Camel | 1,012 (38%) | 1,648 (62%) | 2,660 (7.6%) | 1,575 | 59.2% |
| Total | 14,280 (40.5%) | 20,901 (59.5%) | 35,181 (100%) | 10,893 | 31.0% |
| Species | Herd size prior to outbreak | Herd size after | Deaths | % (-ve) change |
|---|---|---|---|---|
| Camels | 4235 | 2735 | 1575 | 37.2 |
| Cattle | 7360 | 4263 | 3097 | 42.1 |
| Goats | 19532 | 16046 | 3486 | 17.8 |
| Sheep | 14737 | 12172 | 2735 | 18.5 |
| Characteristic | Total N=384 | Laisamis n (%) | Logologo n (%) |
|---|---|---|---|
| Symptom | |||
| Stormy Abortions | 382 (99.5%) | 190 (49.4%) | 192 (50.3%) |
| Bleeding | 157 (40.9%) | 4 (2.5%) | 153 (97.5%) |
| Neurological signs | 111 (28.9%) | 5 (4.5%) | 106 (95.5%) |
| Reasons for keeping livestock | |||
| Sale | 368 (96%) | 180 (48.9%) | 188 (51.1%) |
| Slaughter | 375 (97.9%) | 190 (50.6%) | 185 (49.4%) |
| Milk production | 380 (98.9%) | 150 (39.7%) | 230 (60.3%) |
| Prestige | 0 | 0 | 0 |
| Not sure | 0 | 0 | 0 |
| Protection measures taken | |||
| Yes | 336 (87.5%) | 189 (56.2%) | 147 (43.8%) |
| Types of protective measures taken | |||
| Treat themselves | 380 (98.9%) | 191 (50.3%) | 189 (49.7%) |
| Veterinarian | 52 (13.5%) | 0 (0.0%) | 52 (100%) |
| Herbal treated | 34 (8.8%) | 2 (5.8%) | 32 (94.2%) |
| Vaccinated | 24 (6.2%) | 1 (4.1%) | 23 (95.9%) |
| Species | Losses due to livestock mortality | Milk losses due to mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Category | Price (Ksh)/species | Total Direct Losses (Ksh) | Number of Lactating Dead Females | Price/liter (Ksh) | Liters/day | Duration (Days) | Total (Ksh) | |
| Camels | Adult Male | 60,000 | 20,100,000 | |||||
| Adult Female | 50,000 | 32,500,000 | 399 | 80 | 4 | 60 | 7,660,800 (59.6%) | |
| Young ones | 15,000 | 8,850,000 | ||||||
| Subtotal (1575) | 61,450,000 (38.9%) | |||||||
| Cattle | Adult Male | 50,000 | 10,700,000 | |||||
| Adult Female | 30,000 | 58,680,000 | 253 | 70 | 3 | 60 | 3,187,800 (24.8%) | |
| Young ones | 15,000 | 13,905,000 | ||||||
| Subtotal (3097) | 83,285,000 (52.8%) | |||||||
| Goats | Adult Male | 5,000 | 2,490,000 | |||||
| Adult Female | 3,500 | 3,447,500 | 671 | 100 | 0.5 | 60 | 2,013,000 (15.7%) | |
| Young ones | 500 | 1,001,500 | ||||||
| Subtotal (3486) | 6,939,000 (4.4%) | |||||||
| Sheep | Adult Male | 4,000 | 1,068,000 | |||||
| Adult Female | 3,500 | 4,438,000 | 1143 | N/A | N/A | N/A | N/A | |
| Young ones | 500 | 600,000 | ||||||
| Subtotal (2735) | 6,106,000 (3.9%) | |||||||
| Total (10,893) | 157,780,000 | 12,861,600 | ||||||
| Grand Total (Ksh) | 170,641,600 | |||||||
Ksh: Kenya Shillings
| Category | Amount (KSH) |
|---|---|
| Mortality losses | 157,780,000 (80%) |
| Milk losses due to mortality | 12,861,600 (6.6%) |
| Abortion losses – abortus/fetus | 10,966,500 (5.5%) |
| Subtotal (Direct loss) | 181,608,100 (92.1%) |
| Potential milk losses from aborting animals | 14,580,000 (7.4%) |
| Other losses/expenses* | 1,069,000 (0.5%) |
| Subtotal (Indirect loss) | 15,649,000 (7.9%) |
| Total | 197,257,100 |
*Other losses include: consultation costs, purchase of drugs, slaughtering or burying sick animal carcasses
Ksh: Kenya Shillings
| Species | Losses due to abortions | Potential milk losses due to abortions | Total losses due to abortions and milk losses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | Cost (Ksh)/abortion | Total loss (Ksh) | Number | Price/litre (Ksh) | Litre/day | Days of lactation | Total loss (Ksh) | ||
| Camel | 381 (11.7%) | 15,000 | 5,715,000 (52.1%) | 381 | 80 | 4 | 60 | 7,315,200 (50.1%) | 13,030,200 |
| Cattle | 263 (8.1%) | 15,000 | 3,945,000 (36.0%) | 263 | 70 | 3 | 60 | 3,313,800 (22.7%) | 7,258,800 |
| Goats | 1317 (40.4%) | 500 | 658,500 (6.0%) | 1317 | 100 | 0.5 | 60 | 3,951,000 (27.1%) | 4,609,500 |
| Sheep | 1296 (39.8%) | 500 | 648,000 (5.9%) | 1296 | 0 | 0.5 | 60 | 0 (0.0%) | 648,000 |
| Total | 3257 | 10,966,500 | 14,580,000 | 25,546,500 | |||||
Ksh: Kenya Shillings
| Variables | Categories | Economic loss n (%) | Odds Ratio | 95% Confidence Interval | P value |
|---|---|---|---|---|---|
| Age groups (years) | 10 – 19 | 7 (5.3) | 15.4 | 2.4-97.7 | (0.004) |
| 20 -29 | 28 (21.4) | 2.41 | 0.83-7.08 | (0.108) | |
| 30 -39 | 13 (9.9) | 1.27 | 0.40 – 4.02 | (0.683) | |
| 40 -49 | 63 (48.1) | 3.38 | 1.21-9.42 | (0.019) | |
| 50-59 | 15 (11.4) | 1.29 | 0.42-4.00 | 0.654 | |
| 60+ | 5 (3.8) | Reference | constant | ||
| Sex | Male | 60 (45.8) | 2.39 | 1.54-3.73 | 0.001 |
| Female | 71(54.2) | Reference | |||
| Marital status | Married | 107(81.7) | Reference | Constant | |
| Widow | 4 (3.0) | 0.15 | 0.05-0.42 | 0.0004 | |
| Divorced | 5 (3.8) | 1.28 | 0.39-4.11 | 0.684 | |
| Single | 15 (11.4) | 3.83 | 1.51-9.67 | 0.05 | |
| Religion | Christian | 122 (93.1) | Reference | Constant | |
| Muslim | 9 (6.9) | 1.18 | 0.51-2.74 | 0.707 | |
| Others | 0 | 0.08 | 0.005-1.421 | 0.086 | |
| Education level | No education | 82 (62.6) | Reference | Constant | |
| Primary education | 12 (9.2) | 0.58 | 0.29-1.16 | 0.12 | |
| Secondary | 16 (12.2) | 1.47 | 0.73-2.95 | 0.28 | |
| College | 13 (9.9) | 1.19 | 0.57-2.49 | 0.63 | |
| Adult education | 8 (6.1) | 16.19 | 1.99-131.68 | 0.009 | |
| Occupation | Livestock keeper | 106 (80.9) | Reference | Constant | |
| Crop Farming | 0 (0) | 0.13 | 0.007-2.189 | 0.155 | |
| Self-employed Business | 14 (10.7) | 2.81 | 1.26-6.27 | 0.014 | |
| Student | 11 (8.4) | 3.78 | 1.43-10.02 | 0.008 | |


References
- Lubisi BA, Ndouvhada PN, Neiffer D, Penrith ML, Sibanda DR, Bastos AD. Evaluation of a virus neutralisation test for detection of Rift Valley fever antibodies in suid sera. Trop Med Infect Dis. 2019;4(1):52. Available from: https://www.mdpi.com/2414-6366/4/1/52. doi:10.3390/tropicalmed4010052.
- Ikegami T, Makino S. The pathogenesis of Rift Valley fever. Viruses. 2011;3(5):493-519. Available from: https://www.mdpi.com/1999-4915/3/5/493. doi:10.3390/v3050493.
- Salmon GR, MacLeod M, Claxton JR, Pica Ciamarra U, Robinson T, Duncan A, Peters AR. Exploring the landscape of livestock ‘Facts’. Glob Food Sec. 2020;25:100329. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2211912419300641. doi:10.1016/j.gfs.2019.100329.
- Ould ML, Wafi SE, Fall-Malick FZ, Boushab BM, Ghaber SM, Mokhtar A. Formes hémorragiques graves de la fièvre de la Vallée du Rift: à propos de 5 cas. Pan Afr Med J. 2016;24:73. French. Available from: http://www.panafrican-med-journal.com/content/article/24/73/full/. doi:10.11604/pamj.2016.24.73.9573.
- Boushab BM, Savadogo M, Sow SM, Soufiane S. Enquête d’investigation sur des cas de fièvre de la Vallée du Rift au Tagant, Mauritanie. Rev Epidemiol Sante Publique. 2015;63(3):213-6. French. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0398762015002928. doi:10.1016/j.respe.2015.03.124.
- Ramadan OP, Berta KK, Wamala JF, Maleghemi S, Rumunu J, Ryan C, et al. Analysis of the 2017-2018 Rift Valley fever outbreak in Yirol East County, South Sudan: a one health perspective. Pan Afr Med J. 2022;42(Suppl 1):5. Available from: https://www.panafrican-med-journal.com/content/series/42/1/5/full/. doi:10.11604/pamj.supp.2022.42.1.33769.
- Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Depner K, Drewe JA, Garin-Bastuji B, Rojas JL, Schmidt CG, Michel V, et al. Rift Valley fever – epidemiological update and risk of introduction into Europe. EFSA J. 2020;18(3):e06041. Available from: https://data.europa.eu/doi/10.2903/j.efsa.2020.6041. doi:10.2903/j.efsa.2020.6041.
- De Glanville WA, Allan KJ, Nyarobi JM, Thomas KM, Lankester F, Kibona TJ, Claxton JR, Brennan B, Carter RW, Crump JA, et al. An outbreak of Rift Valley fever among peri-urban dairy cattle in northern Tanzania. Trans R Soc Trop Med Hyg. 2022;116(11):1082-90. Available from: https://academic.oup.com/trstmh/article/116/11/1082/6679090. doi:10.1093/trstmh/trac076.
- Nanyingi MO, Munyua P, Kiama SG, Muchemi GM, Thumbi SM, Bitek AO, Bett B, Muriithi RM, Njenga MK. A systematic review of Rift Valley Fever epidemiology 1931–2014. Infect Ecol Epidemiol. 2015;5:28024. Available from: https://www.tandfonline.com/doi/full/10.3402/iee.v5.28024. doi:10.3402/iee.v5.28024.
- Mosomtai G, Evander M, Sandström P, Ahlm C, Sang R, Hassan OA, Affognon H, Landmann T. Association of ecological factors with Rift Valley fever occurrence and mapping of risk zones in Kenya. Int J Infect Dis. 2016;46:49-55. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1201971216310001. doi:10.1016/j.ijid.2016.03.013.
- Gikungu D, Wakhungu J, Siamba D, Neyole E, Muita R, Bett B. Dynamic risk model for Rift Valley fever outbreaks in Kenya based on climate and disease outbreak data. Geospat Health. 2016;11(2):377. Available from: http://www.geospatialhealth.net/index.php/gh/article/view/377. doi:10.4081/gh.2016.377.
- Chengula AA, Mdegela RH, Kasanga CJ. Socio-economic impact of Rift Valley fever to pastoralists and agro pastoralists in Arusha, Manyara and Morogoro regions in Tanzania. Springerplus. 2013;2:549. Available from: https://springerplus.springeropen.com/articles/10.1186/2193-1801-2-549. doi:10.1186/2193-1801-2-549.
- Sang R, Arum S, Chepkorir E, Mosomtai G, Tigoi C, Sigei F, Lwande OW, Landmann T, Affognon H, Ahlm C, et al. Distribution and abundance of key vectors of Rift Valley fever and other arboviruses in two ecologically distinct counties in Kenya. PLoS Negl Trop Dis. 2017;11(2):e0005341. Bird B, editor. Available from: https://dx.plos.org/10.1371/journal.pntd.0005341. doi:10.1371/journal.pntd.0005341.
- Liu B, Ma J, Jiao Z, Gao X, Xiao J, Wang H. Risk assessment for the Rift Valley fever occurrence in China: special concern in southwest border areas. Transbound Emerg Dis. 2021;68(2):445-57. Available from: https://onlinelibrary.wiley.com/doi/10.1111/tbed.13695. doi:10.1111/tbed.13695.
- Anderson A, Shwiff S, Gebhardt K, Ramírez AJ, Shwiff S, Kohler D, Lecuona L. Economic evaluation of vampire bat (Desmodus rotundus) rabies prevention in Mexico. Transbound Emerg Dis. 2014;61(2):140-6. Available from: https://onlinelibrary.wiley.com/doi/10.1111/tbed.12007. doi:10.1111/tbed.12007.
- Muga GO, Onyango-Ouma W, Sang R, Affognon H. Sociocultural and economic dimensions of Rift Valley fever. Am J Trop Med Hyg. 2015;92(4):730-8. Available from: https://www.ajtmh.org/view/journals/tpmd/92/4/article-p730.xml. doi:10.4269/ajtmh.14-0363.
- Marsh TL, Yoder J, Deboch T, McElwain TF, Palmer GH. Livestock vaccinations translate into increased human capital and school attendance by girls. Sci Adv. 2016;2(12):e1601410. Available from: https://www.science.org/doi/10.1126/sciadv.1601410. doi:10.1126/sciadv.1601410.
- Zimbini M, Cynthia BN. Evaluating the impact of 2010 Rift Valley fever outbreaks on sheep numbers in three provinces of South Africa. Afr J Agric Res. 2017;12(12):979-86. Available from: http://academicjournals.org/journal/AJAR/article-abstract/082D44063274. doi:10.5897/AJAR2016.11130.
- Jones BA, Muhammed A, Ali ET, Homewood KM, Pfeiffer DU. Pastoralist knowledge of sheep and goat disease and implications for peste des petits ruminants virus control in the Afar Region of Ethiopia. Prev Vet Med. 2020;174:104808. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167587719301011. doi:10.1016/j.prevetmed.2019.104808.
- Marimwe MC, Fosgate GT, Roberts LC, Tavornpanich S, Olivier AJ, Abolnik C. The spatiotemporal epidemiology of high pathogenicity avian influenza outbreaks in key ostrich producing areas of South Africa. Prev Vet Med. 2021;196:105474. Available from: https://linkinghub.elsevier.com/retrieve/pii/S016758772100218X. doi:10.1016/j.prevetmed.2021.105474.
- Himeidan YE, Kweka EJ, Mahgoub MM, El Rayah EA, Ouma JO. Recent outbreaks of Rift Valley fever in East Africa and the Middle East. Front Public Health. 2014;2:169. Available from: http://journal.frontiersin.org/article/10.3389/fpubh.2014.00169/abstract. doi:10.3389/fpubh.2014.00169.
- Kenya National Bureau of Statistics. 2019 Kenya population and housing census: main reports [Internet]. Nairobi: Kenya National Bureau of Statistics; 2019 Nov. Available from: https://www.knbs.or.ke/reports/kenya-census-2019/.
- Oyas H, Holmstrom L, Kemunto NP, Muturi M, Mwatondo A, Osoro E, Bitek A, Bett B, Githinji JW, Thumbi SM, et al. Enhanced surveillance for Rift Valley fever in livestock during El Niño rains and threat of RVF outbreak, Kenya, 2015-2016. PLoS Negl Trop Dis. 2018;12(4):e0006353. Smith D, editor. Available from: https://dx.plos.org/10.1371/journal.pntd.0006353. doi:10.1371/journal.pntd.0006353.
- Budasha NH, Gonzalez JP, Sebhatu TT, Arnold E. Rift Valley fever seroprevalence and abortion frequency among livestock of Kisoro district, South Western Uganda (2016): a prerequisite for zoonotic infection. BMC Vet Res. 2018;14(1):271. Available from: https://bmcvetres.biomedcentral.com/articles/10.1186/s12917-018-1596-8. doi:10.1186/s12917-018-1596-8.
- Page SW, Gautier P. Use of antimicrobial agents in livestock. Rev Sci Tech. 2012;31(1):145-88. Available from: https://doc.woah.org/dyn/portal/digidoc.xhtml?statelessToken=ve1nKAjSnnIp05R-csC3ta5-zIu4yXaWZeFdHPOIYlc=&actionMethod=dyn%2Fportal%2Fdigidoc.xhtml%3AdownloadAttachment.openStateless. doi:10.20506/rst.31.1.2106.
- Gachohi JM, Njenga MK, Kitala P, Bett B. Modelling vaccination strategies against Rift Valley fever in livestock in Kenya. PLoS Negl Trop Dis. 2016;10(12):e0005049. Diemert DJ, editor. Available from: https://dx.plos.org/10.1371/journal.pntd.0005049. doi:10.1371/journal.pntd.0005049. Erratum in: Gachohi JM, Njenga MK, Kitala P, Bett B. Correction: modelling vaccination strategies against Rift Valley fever in livestock in Kenya. PLoS Negl Trop Dis. 2017;11(1):e0005316. Available from: https://dx.plos.org/10.1371/journal.pntd.0005316.
- Dungu B, Lubisi BA, Ikegami T. Rift Valley fever vaccines: current and future needs. Curr Opin Virol. 2018;29:8-15. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1879625718300026. doi:10.1016/j.coviro.2018.02.001.
- Dungu B, Donadeu M, Bouloy M. Vaccination for the control of Rift Valley fever in enzootic and epizootic situations. Dev Biol (Basel). 2013;135:61-72. Available from: https://karger.com/chapter/doi/10.1159/000157178. doi:10.1159/000157178.
- El Mamy AB, Baba MO, Barry Y, Isselmou K, Dia ML, Hampate B, Diallo MY, El Kory MO, Diop M, Lo MM, et al. Unexpected Rift Valley fever outbreak, northern Mauritania. Emerg Infect Dis. 2011;17(10):1894-6. Available from: http://wwwnc.cdc.gov/eid/article/17/10/11-0397_article.htm. doi:10.3201/eid1710.110397.
- Pendell DL, Lusk JL, Marsh TL, Coble KH, Szmania SC. Economic assessment of zoonotic diseases: an illustrative study of Rift Valley fever in the United States. Transbound Emerg Dis. 2016;63(2):203-14. Available from: https://onlinelibrary.wiley.com/doi/10.1111/tbed.12246. doi:10.1111/tbed.12246.
- Peyre M, Chevalier V, Abdo-Salem S, Velthuis A, Antoine-Moussiaux N, Thiry E, Roger F. A systematic scoping study of the socio-economic impact of Rift Valley fever: research gaps and needs. Zoonoses Public Health. 2015;62(5):309-25. Available from: https://onlinelibrary.wiley.com/doi/10.1111/zph.12153. doi:10.1111/zph.12153.
- Tinto B, Quellec J, Cêtre-Sossah C, Dicko A, Salinas S, Simonin Y. Rift Valley fever in West Africa: a zoonotic disease with multiple socio-economic consequences. One Health. 2023;17:100583. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2352771423001039. doi:10.1016/j.onehlt.2023.100583.
- Sindato C, Karimuribo ED, Pfeiffer DU, Mboera LE, Kivaria F, Dautu G, Bernard B, Paweska JT. Spatial and temporal pattern of Rift Valley fever outbreaks in Tanzania; 1930 to 2007. PLoS One. 2014;9(2):e88897. Ikegami T, editor. Available from: https://dx.plos.org/10.1371/journal.pone.0088897. doi:10.1371/journal.pone.0088897.
- Ng’ang’a CM, Bukachi SA, Bett BK. Lay perceptions of risk factors for Rift Valley fever in a pastoral community in northeastern Kenya. BMC Public Health. 2016;16:32. Available from: http://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-016-2707-8. doi:10.1186/s12889-016-2707-8.
- Wanyoike F, Rich KM. An assessment of the regional and national socio-economic impacts of the 2007 Rift Valley fever outbreak in Kenya. Am J Trop Med Hyg. 2010;83(2 Suppl):52-7. Available from: https://ajtmh.org/doi/10.4269/ajtmh.2010.09-0291. doi:10.4269/ajtmh.2010.09-0291.
- Hassan OA, Ahlm C, Sang R, Evander M. The 2007 Rift Valley fever outbreak in Sudan. PLoS Negl Trop Dis. 2011;5(9):e1229. Brooker S, editor. Available from: https://dx.plos.org/10.1371/journal.pntd.0001229. doi:10.1371/journal.pntd.0001229.
- Rasmussen P, Shaw AP, Jemberu WT, Knight-Jones T, Conrady B, Apenteng OO, Cheng Y, Muñoz V, Rushton J, Torgerson PR. Economic losses due to foot-and-mouth disease (FMD) in Ethiopian cattle. Prev Vet Med. 2024;230:106276. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0167587724001624. doi:10.1016/j.prevetmed.2024.106276.
